Vascular Cognitive Impairment and Dementia
- PMID: 35678401
- PMCID: PMC9833847
- DOI: 10.1212/CON.0000000000001124
Vascular Cognitive Impairment and Dementia
Abstract
Purpose of review: This article gives a broad overview of vascular cognitive impairment and dementia, including epidemiology, pathophysiology, clinical approach, and management. Emphasis is placed on understanding the common underlying types of cerebrovascular disease (including atherosclerosis, arteriolosclerosis, and cerebral amyloid angiopathy) and awareness of rare inherited cerebrovascular disorders.
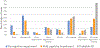
Recent findings: The pathophysiology of vascular cognitive impairment and dementia is heterogeneous, and the most recent diagnostic criteria for vascular cognitive impairment and dementia break down the diagnosis of major vascular dementia into four phenotypic categories, including subcortical ischemic vascular dementia, poststroke dementia, multi-infarct dementia, and mixed dementia. Control of cardiovascular risk factors, including management of midlife blood pressure, cholesterol, and blood sugars, remains the mainstay of prevention for vascular cognitive impairment and dementia. Cerebral amyloid angiopathy requires special consideration when it comes to risk factor management given the increased risk of spontaneous intracerebral hemorrhage. Recent trials suggest some improvement in global cognitive function in patients with vascular cognitive impairment and dementia with targeted cognitive rehabilitation.
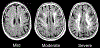
Summary: Thorough clinical evaluation and neuroimaging form the basis for diagnosis. As vascular cognitive impairment and dementia is the leading nondegenerative cause of dementia, identifying risk factors and optimizing their management is paramount. Once vascular brain injury has occurred, symptomatic management should be offered and secondary prevention pursued.
Copyright © 2022 American Academy of Neurology.
Figures





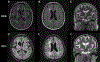
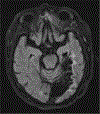


References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th ed. American Psychiatric Association; 2013.
-
- Willis T De anima brutorum quae hominis vitalis ac sentitiva est: exercitationes duae. Typis E.F. impensis Ric Davis, Oxon; 1672.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
