Understanding barriers to diagnosis in a rare, genetic disease: Delays and errors in diagnosing schwannomatosis
- PMID: 35678462
- PMCID: PMC9378587
- DOI: 10.1002/ajmg.a.62860
Understanding barriers to diagnosis in a rare, genetic disease: Delays and errors in diagnosing schwannomatosis
Abstract
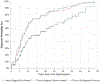
Diagnosis of rare, genetic diseases is challenging, but conceptual frameworks of the diagnostic process can guide quality improvement initiatives. Using the National Academy of Medicine diagnostic framework, we assessed the extent of, and reasons for diagnostic delays and diagnostic errors in schwannomatosis, a neurogenetic syndrome characterized by nerve sheath tumors and chronic pain. We reviewed the medical records of 97 people with confirmed or probable schwannomatosis seen in two US tertiary care clinics. Time-to-event analysis revealed a median time from first symptom to diagnosis of 16.7 years (95% CI, 7.5-26.0 years) and median time from first medical consultation to diagnosis of 9.8 years (95% CI, 3.5-16.2 years). Factors associated with longer times to diagnosis included initial signs/symptoms that were intermittent, non-specific, or occurred at younger ages (p < 0.05). Thirty-six percent of patients were misdiagnosed; misdiagnoses were of underlying genetic condition (18.6%), pain etiology (16.5%), and nerve sheath tumor presence/pathology (11.3%) (non-mutually exclusive categories). One-fifth (19.6%) of patients had a clear missed opportunity for genetics workup that could have led to an earlier schwannomatosis diagnosis. These results suggest that interventions in clinician education, genetic testing availability, expert review of pathology findings, and automatic triggers for genetics referrals may improve diagnosis of schwannomatosis.
Keywords: delayed diagnosis; diagnostic errors; missed diagnosis; rare disease; schwannomatosis.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
Conflicts of Interest: Vanessa Merker reports consulting income from the Neurofibromatosis Network. Justin Jordan reports consulting income from Navio Theragnostics, CereXis, Recursion, Health2047; <1% stock ownership in Navio Theragnostics; Royalties from Elsevier. Jaishri Blakeley has served as a paid consultant for Astella Pharma, Abbvie, TriAct/BiPar Sciences and has had research support from GlaxoSmithKlein, Bristol Meyer Squibb, and AstraZeneca. Scott Plotkin is co-founder of NFlection Therapeutics and NF2 Therapeutics; serves on the Scientific Advisory Board and has stock in SonALAsense; and consults for Akouos.
Figures


References
-
- EURORDIS. The Voice of 12,000 Patients: Experiences and Expectations of Rare Disease Patients on Diagnosis and Care in Europe. 2009. (http://www.eurordis.org/IMG/pdf/voice_12000_patients/EURORDISCARE_FULLBO....
-
- Shire Genetic Therapies. Rare Disease Impact Report: Insights from patients and the medical community. April 2013. Available at https://globalgenes.org/wp-content/uploads/2013/04/ShireReport-1.pdf.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

