Mechanosensitive Steroid Hormone Signaling and Cell Fate
- PMID: 35678467
- PMCID: PMC9237634
- DOI: 10.1210/endocr/bqac085
Mechanosensitive Steroid Hormone Signaling and Cell Fate
Abstract
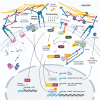
Mechanical forces collaborate across length scales to coordinate cell fate during development and the dynamic homeostasis of adult tissues. Similarly, steroid hormones interact with their nuclear and nonnuclear receptors to regulate diverse physiological processes necessary for the appropriate development and function of complex multicellular tissues. Aberrant steroid hormone action is associated with tumors originating in hormone-sensitive tissues and its disruption forms the basis of several therapeutic interventions. Prolonged perturbations to mechanical forces may further foster tumor initiation and the evolution of aggressive metastatic disease. Recent evidence suggests that steroid hormone and mechanical signaling intersect to direct cell fate during development and tumor progression. Potential mechanosensitive steroid hormone signaling pathways along with their molecular effectors will be discussed in this context.
Keywords: cell fate; mechanosensitive; mechanotransduction; reproductive cancers; steroid hormones; tissue mechanics.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677-689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

