MicroRNA 320a and Membrane Antigens as Tools to Evaluate the Pathophysiology of Platelets Stored in Blood Banks
- PMID: 35678655
- PMCID: PMC9164066
- DOI: 10.3390/cimb44050126
MicroRNA 320a and Membrane Antigens as Tools to Evaluate the Pathophysiology of Platelets Stored in Blood Banks
Abstract
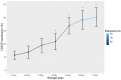
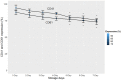
Our research group, through the analysis of miRNomes in platelet concentrates (PCs) stored in blood banks, identified and validated the miR-127 and miR-320a miRNAs as biomarkers of platelet storage lesions (PSLs) in PCs. In order to validate the miRNAs 127 and 320a methodologically, as PSL biomarkers in a large number of PC bags, we also evaluated important immunological markers involved in the platelet activation/aggregation process-the CD62P receptor (P-selectin), the surface glycoproteins (GP) IIb/IIIa, and the purinergic P2Y12 receptor-via flow cytometry. The miRNAs miR-127 and miR-320a were quantified by real-time quantitative PCR (RT-qPCR). To carry out this study, 500 collection tubes were used at the upper edge of the PC bags containing platelets. Each tube was divided into seven equal parts (totaling 3500 samples) for platelet analysis from 7 different storage days, where the 1st day represents the high-quality control, and the 7th day corresponds to the low-quality control of the platelets. After analyzing all parameters during storage days, it was concluded that the relative quantification of miR-320a below 0.50 and the CD62P receptor below 27.92% are reliable indicators of the absence of storage lesions in blood banks. We believe that the values found in the expression of the CD62P receptor legitimize the use of the miR-320a and miR-127 miRNAs to build a kit capable of accurately measuring whether the stored platelets are suitable for transfusion.
Keywords: biomarkers; membrane antigens; miR-127; miR-320a; platelet concentrate; storage lesion.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Canault M., Duerschmied D., Brill A., Stefanini L., Schatzberg D., Cifuni S.M., Bergmeier W., Wagner D.D. p38 mitogen-activated protein kinase activation during platelet storage: Consequences for platelet recovery and hemostatic function in vivo. Blood. 2010;115:1835–1842. doi: 10.1182/blood-2009-03-211706. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

