Role of hair follicles in the pathogenesis of arsenical-induced cutaneous damage
- PMID: 35678766
- PMCID: PMC9531897
- DOI: 10.1111/nyas.14809
Role of hair follicles in the pathogenesis of arsenical-induced cutaneous damage
Abstract
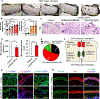
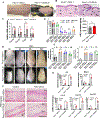
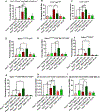
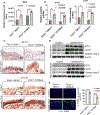
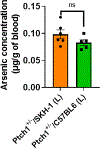
Arsenical vesicants cause skin inflammation, blistering, and pain. The lack of appropriate animal models causes difficulty in defining their molecular pathogenesis. Here, Ptch1+/- /C57BL/6 mice were employed to investigate the pathobiology of the arsenicals lewisite and phenylarsine oxide (PAO). Following lewisite or PAO challenge (24 h), the skin of animals becomes grayish-white, thick, leathery, and wrinkled with increased bi-fold thickness, Draize score, and necrotic patches. In histopathology, infiltrating leukocytes (macrophages and neutrophils), epidermal-dermal separation, edema, apoptotic cells, and disruption of tight and adherens junction proteins can be visualized. PCR arrays and nanoString analyses showed significant increases in cytokines/chemokines and other proinflammatory mediators. As hair follicles (HFs), which provide an immune-privileged environment, may affect immune cell trafficking and consequent inflammatory responses, we compared the pathogenesis of these chemicals in this model to that in Ptch1+/- /SKH-1 hairless mice. Ptch1+/- /SKH-1 mice have rudimentary, whereas Ptch1+/- /C57BL/6 mice have well-developed HFs. Although no significant differences were observed in qualitative inflammatory responses between the two strains, levels of cytokines/chemokines differed. Importantly, the mechanism of inflammation was identical; both reactive oxygen species induction and consequent activation of unfolded protein response signaling were similar. These data reveal that the acute molecular pathogenesis of arsenicals in these two murine models is similar.
Keywords: animal model; arsenicals; hair follicle; skin injury; vesicants.
© 2022 New York Academy of Sciences.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing interests.
Figures


Similar articles
-
Common molecular profile of multiple structurally distinct warfare arsenicals in causing cutaneous chemical vesicant injury.Sci Rep. 2025 Feb 22;15(1):6505. doi: 10.1038/s41598-024-83513-1. Sci Rep. 2025. PMID: 39987158 Free PMC article.
-
Molecular Mechanism Underlying Pathogenesis of Lewisite-Induced Cutaneous Blistering and Inflammation: Chemical Chaperones as Potential Novel Antidotes.Am J Pathol. 2016 Oct;186(10):2637-49. doi: 10.1016/j.ajpath.2016.06.012. Epub 2016 Aug 12. Am J Pathol. 2016. PMID: 27528504 Free PMC article.
-
Cutaneous exposure to lewisite causes acute kidney injury by invoking DNA damage and autophagic response.Am J Physiol Renal Physiol. 2018 Jun 1;314(6):F1166-F1176. doi: 10.1152/ajprenal.00277.2017. Epub 2018 Jan 17. Am J Physiol Renal Physiol. 2018. PMID: 29361668 Free PMC article.
-
Biological and environmental hazards associated with exposure to chemical warfare agents: arsenicals.Ann N Y Acad Sci. 2016 Aug;1378(1):143-157. doi: 10.1111/nyas.13214. Epub 2016 Sep 16. Ann N Y Acad Sci. 2016. PMID: 27636894 Free PMC article. Review.
-
The role of the microbiome in scalp hair follicle biology and disease.Exp Dermatol. 2020 Mar;29(3):286-294. doi: 10.1111/exd.13935. Epub 2019 May 15. Exp Dermatol. 2020. PMID: 30974503 Review.
Cited by
-
Effects of Mycobacterium vaccae NCTC 11659 and Lipopolysaccharide Challenge on Polarization of Murine BV-2 Microglial Cells.Int J Mol Sci. 2023 Dec 29;25(1):474. doi: 10.3390/ijms25010474. Int J Mol Sci. 2023. PMID: 38203645 Free PMC article.
-
Common molecular profile of multiple structurally distinct warfare arsenicals in causing cutaneous chemical vesicant injury.Sci Rep. 2025 Feb 22;15(1):6505. doi: 10.1038/s41598-024-83513-1. Sci Rep. 2025. PMID: 39987158 Free PMC article.
-
Relevance of the Platelet-activating factor system in chemical warfare agents-induced effects.Free Radic Biol Med. 2025 Feb 16;228:62-67. doi: 10.1016/j.freeradbiomed.2024.12.037. Epub 2024 Dec 18. Free Radic Biol Med. 2025. PMID: 39706499 Review.
-
Dermal Exposure to Vesicating Nettle Agent Phosgene Oxime: Clinically Relevant Biomarkers and Skin Injury Progression in Murine Models.J Pharmacol Exp Ther. 2024 Jan 17;388(2):536-545. doi: 10.1124/jpet.123.001718. J Pharmacol Exp Ther. 2024. PMID: 37652710 Free PMC article.
-
HSP90, a Common Therapeutic Target for Suppressing Skin Injury Caused by Exposure to Chemically Diverse Classes of Blistering Agents.J Pharmacol Exp Ther. 2024 Jan 17;388(2):546-559. doi: 10.1124/jpet.123.001795. J Pharmacol Exp Ther. 2024. PMID: 37914412 Free PMC article.
References
-
- Vilches D, Alburquerque G & Ramirez-Tagle R. 2016. One hundred and one years after a milestone: Modern chemical weapons and World War I. Educación Química. 27: 233–236.
-
- “Chemical Weapons. https://www.un.org/disarmament/wmd/chemical/“. In. United Nations.
-
- Riddle JR, Brown M, Smith T, et al. 2003. Chemical warfare and the Gulf War: a review of the impact on Gulf veterans’ health. Mil Med. 168: 606–613. - PubMed
-
- Watch HR 2018. Syria: A Year On, Chemical Weapons Attacks Persist. https://www.hrw.org/news/2018/04/04/syria-year-chemical-weapons-attacks-... [Accessed April 4, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

