Effectiveness and Safety of Tofacitinib in Canadian Patients With Rheumatoid Arthritis: Primary Results From a Prospective Observational Study
- PMID: 35678771
- PMCID: PMC10091934
- DOI: 10.1002/acr.24966
Effectiveness and Safety of Tofacitinib in Canadian Patients With Rheumatoid Arthritis: Primary Results From a Prospective Observational Study
Abstract
Objective: The Canadian Tofacitinib for Rheumatoid Arthritis Observational (CANTORAL) is the first Canadian prospective, observational study assessing tofacitinib. The objective was to assess effectiveness and safety for moderate to severe rheumatoid arthritis (RA). Coprimary and secondary outcomes are reported from an interim analysis.
Methods: Patients initiating tofacitinib from October 2017 to July 2020 were enrolled from 45 Canadian sites. Coprimary outcomes (month 6) included the Clinical Disease Activity Index (CDAI)-defined low disease activity (LDA) and remission. Secondary outcomes (to month 18) included the CDAI and the 4-variable Disease Activity Score in 28 joints (DAS28) using the erythrocyte sedimentation rate (ESR)/C-reactive protein (CRP) level to define LDA and remission; the proportions of patients achieving mild pain (visual analog scale <20 mm), and moderate (≥30%) and substantial (≥50%) pain improvements; and the proportions of patients achieving a Health Assessment Questionnaire disability index (HAQ DI) score greater or equal to normative values (≤0.25) and a HAQ DI score greater or equal to minimum clinically important difference (MCID) (≥0.22). Safety was assessed to month 36.
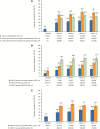
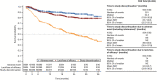
Results: Of 504 patients initiating tofacitinib, 44.4% received concomitant methotrexate. At month 6, 52.9% and 15.4% of patients were in CDAI-defined LDA and remission, respectively; a similar proportion of patients achieved outcomes by month 3 (first post-baseline assessment). By month 3, 27.2% and 41.7% of patients, respectively, were in DAS28-ESR-defined LDA and DAS28-CRP <3.2; 14.7% and 25.8% achieved DAS28-ESR remission and DAS28-CRP <2.6. By month 3, mild pain and moderate and substantial pain improvements occurred in 29.6%, 55.6%, and 42.9% of patients, respectively; 19.9% and 53.7% of patients achieved a HAQ DI score greater than or equal to normative values and a HAQ DI score greater than or equal to MCID, respectively. Outcomes were generally maintained to month 18. Incidence rates (events per 100 patient-years) for treatment-emergent adverse events (AEs), serious AEs, and discontinuations due to AEs were 126.8, 11.9, and 14.5, respectively, and AEs of special interest were infrequent.
Conclusion: Tofacitinib was associated with early and sustained improvement in RA signs and symptoms in real-world patients. Effectiveness and safety were consistent with the established tofacitinib clinical profile.
© 2022 Pfizer Inc. Arthritis Care & Research published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

References
-
- Bykerk VP, Akhavan P, Hazlewood GS, et al. Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease‐modifying antirheumatic drugs. J Rheumatol 2012;39:1559–82. - PubMed
-
- Smolen JS, Landewé RB, Bijlsma JW, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. - PubMed
-
- Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2021;73:1108–23. - PubMed
-
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376:1094–108. - PubMed
-
- Pfizer Canada Inc . Xeljanz (product monograph). 2019. URL: https://www.pfizer.ca/sites/default/files/202206/Xeljanz_PM_EN_258173_09....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

