Brain infiltration of breast cancer stem cells is facilitated by paracrine signaling by inhibitor of differentiation 3 to nuclear respiratory factor 1
- PMID: 35678885
- PMCID: PMC11801135
- DOI: 10.1007/s00432-022-04026-w
Brain infiltration of breast cancer stem cells is facilitated by paracrine signaling by inhibitor of differentiation 3 to nuclear respiratory factor 1
Abstract
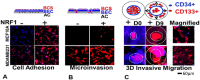
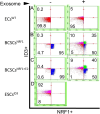
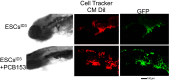
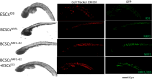
Treatment options for brain metastatic breast cancer are limited because the molecular mechanism for how breast cancer cells infiltrate the brain is not fully understood. For breast tumors to metastasize to the brain first, cells need to detach from the primary tumor, enter in the blood circulation, survive within the microvascular niche, and then cross the blood-brain barrier (BBB) to colonize into the brain. It is critical to understand how breast cancer cells transmigrate through the BBB to prevent brain metastasis. Nuclear respiratory factor 1 (NRF1) transcription factor has been reported to be highly active in several human cancers and its aberrant expression facilitates in the acquisition of breast cancer stem cells (BCSCs). Inhibitor of differentiation protein 3 (ID3), a transcription regulating protein, induces pluripotent endothelial stem cells (ESCs). Herein, we investigated if NRF1-induced BCSCs could cross a BBB model and guiding of BCSCs by ID3-induced ESCs across the BBB. BCSCs and ESCs were subjected to functional gain/loss experiments to determine if NRF1/ID3 contributed to lineage-specific BCSCs organ entry. First, we tested whether NRF1 promoted migration of breast cancer using a BBB model consisting of BCSCs or MDA-MB231 cells, brain endothelial cell layer, and astrocytes. NRF1 overexpression increased the propensity for BCSCs and NRF1-induced MDA-MB231 cells to adhere to brain endothelial cells and migrate across a human BBB model. Increased adhesion of NRF1-induced BCSCs to ESCsID3 was detected. NRF1-induced BCSCs crossed through the BBB model and this was promoted by ESCsID3. We also showed that environmental relevant exposure to PCBs (PCB153 and PCB77) produced differential effects. Treatment with PCB153 showed increased growth of NRF1-induced BCSCs tumor spheroids and increased in vivo migration of ESCsID3. Exosomal ID3 released from endothelial cells also supported the growth of NRF1-induced BCSCs and provide the basis for paracrine effects by ESCsID3 associated with breast tumors. Xenograft experiments showed that ID3 overexpressing brain ESCs not only supported the growth of BCSC tumor spheroids but guided them to the neural crest in zebrafish. These findings show for the first time a novel role for ID3 and NRF1 by which ESCsID3 help guide BCSCsNRF1 to distant metastatic sites where they most likely facilitate the colonization, survival, and proliferation of BCSCs. This knowledge is important for pre-clinical testing of NRF1/ID3 modifying agents to prevent the spread of breast cancer to the brain.
Keywords: Breast cancer metastasis; Cancer stem cells; ID3; NRF1.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Authors have no conflicts of interest.
Figures





Similar articles
-
An update on cancer stem cell survival pathways involved in chemoresistance in triple-negative breast cancer.Future Oncol. 2025 Mar;21(6):715-735. doi: 10.1080/14796694.2025.2461443. Epub 2025 Feb 12. Future Oncol. 2025. PMID: 39936282 Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Adapting Safety Plans for Autistic Adults with Involvement from the Autism Community.Autism Adulthood. 2025 May 28;7(3):293-302. doi: 10.1089/aut.2023.0124. eCollection 2025 Jun. Autism Adulthood. 2025. PMID: 40539213
Cited by
-
Molecular signaling network and therapeutic developments in breast cancer brain metastasis.Adv Cancer Biol Metastasis. 2023 Jul;7:100079. doi: 10.1016/j.adcanc.2022.100079. Adv Cancer Biol Metastasis. 2023. PMID: 36536947 Free PMC article.
-
Nuclear respiratory factor 1 promotes the progression of EBV-associated gastric cancer and maintains EBV latent infection.Virus Genes. 2023 Apr;59(2):204-214. doi: 10.1007/s11262-023-01970-8. Epub 2023 Feb 4. Virus Genes. 2023. PMID: 36738378
-
Zebrafish xenografts in breast cancer research.Front Immunol. 2025 Jul 10;16:1540610. doi: 10.3389/fimmu.2025.1540610. eCollection 2025. Front Immunol. 2025. PMID: 40709187 Free PMC article. Review.
References
-
- Chen YH, Wu ZQ, Zhao YL, Si YL, Guo MZ, Han WD (2012) FHL2 inhibits the Id3-promoted proliferation and invasive growth of human MCF-7 breast cancer cells. Chin Med J (Engl) 125:2329–2333 - PubMed
-
- Chu S, Covaci A, Schepens P (2003) Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ Res 93(2):167–176 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

