Human HspB1, HspB3, HspB5 and HspB8: Shaping these disease factors during vertebrate evolution
- PMID: 35678958
- PMCID: PMC9346038
- DOI: 10.1007/s12192-022-01268-y
Human HspB1, HspB3, HspB5 and HspB8: Shaping these disease factors during vertebrate evolution
Abstract
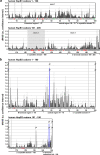
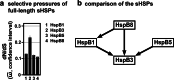
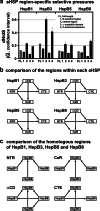
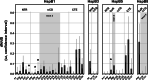
Small heat shock proteins (sHSPs) emerged early in evolution and occur in all domains of life and nearly in all species, including humans. Mutations in four sHSPs (HspB1, HspB3, HspB5, HspB8) are associated with neuromuscular disorders. The aim of this study is to investigate the evolutionary forces shaping these sHSPs during vertebrate evolution. We performed comparative evolutionary analyses on a set of orthologous sHSP sequences, based on the ratio of non-synonymous: synonymous substitution rates for each codon. We found that these sHSPs had been historically exposed to different degrees of purifying selection, decreasing in this order: HspB8 > HspB1, HspB5 > HspB3. Within each sHSP, regions with different degrees of purifying selection can be discerned, resulting in characteristic selective pressure profiles. The conserved α-crystallin domains were exposed to the most stringent purifying selection compared to the flanking regions, supporting a 'dimorphic pattern' of evolution. Thus, during vertebrate evolution the different sequence partitions were exposed to different and measurable degrees of selective pressures. Among the disease-associated mutations, most are missense mutations primarily in HspB1 and to a lesser extent in the other sHSPs. Our data provide an explanation for this disparate incidence. Contrary to the expectation, most missense mutations cause dominant disease phenotypes. Theoretical considerations support a connection between the historic exposure of these sHSP genes to a high degree of purifying selection and the unusual prevalence of genetic dominance of the associated disease phenotypes. Our study puts the genetics of inheritable sHSP-borne diseases into the context of vertebrate evolution.
Keywords: Alpha B-crystallin; Disease-associated missense mutation; Genetic dominance; Genotype-phenotype relationship; Neuropathy, Myopathy; Purifying selection.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
-
- Almeida-Souza L, Goethals S, De WV, Dierick I, Gallardo R, Van DJ, Irobi J, Gettemans J, Rousseau F, Schymkowitz J, Timmerman V, Janssens S. Increased monomerization of mutant HSPB1 leads to protein hyperactivity in Charcot-Marie-Tooth neuropathy. J Biol Chem. 2010;285:12778–12786. doi: 10.1074/jbc.M109.082644. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

