Oxycodone for cancer-related pain
- PMID: 35679121
- PMCID: PMC9180760
- DOI: 10.1002/14651858.CD003870.pub7
Oxycodone for cancer-related pain
Abstract
Background: Many people with cancer experience moderate to severe pain that requires treatment with strong opioids, such as oxycodone and morphine. Strong opioids are, however, not effective for pain in all people, neither are they well tolerated by all people. The aim of this review was to assess whether oxycodone is associated with better pain relief and tolerability than other analgesic options for adults with cancer pain. This is an updated Cochrane review previously published in 2017.
Objectives: To assess the effectiveness and tolerability of oxycodone by any route of administration for pain in adults with cancer.
Search methods: For this update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE and MEDLINE In-Process (Ovid), Embase (Ovid), Science Citation Index, Conference Proceedings Citation Index - Science (ISI Web of Science), BIOSIS (ISI), and PsycINFO (Ovid) to November 2021. We also searched four trial registries, checked the bibliographic references of relevant studies, and contacted the authors of the included studies. We applied no language, date, or publication status restrictions.
Selection criteria: We included randomised controlled trials (parallel-group or cross-over) comparing oxycodone (any formulation or route of administration) with placebo or an active drug (including oxycodone) for cancer background pain in adults by examining pain intensity/relief, adverse events, quality of life, and participant preference.
Data collection and analysis: Two review authors independently sifted the search, extracted data and assessed the included studies using standard Cochrane methodology. We meta-analysed pain intensity data using the generic inverse variance method, and pain relief and adverse events using the Mantel-Haenszel method, or summarised these data narratively along with the quality of life and participant preference data. We assessed the overall certainty of the evidence using GRADE.
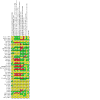
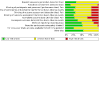
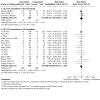
Main results: For this update, we identified 19 new studies (1836 participants) for inclusion. In total, we included 42 studies which enrolled/randomised 4485 participants, with 3945 of these analysed for efficacy and 4176 for safety. The studies examined a number of different drug comparisons. Controlled-release (CR; typically taken every 12 hours) oxycodone versus immediate-release (IR; taken every 4-6 hours) oxycodone Pooled analysis of three of the four studies comparing CR oxycodone to IR oxycodone suggest that there is little to no difference between CR and IR oxycodone in pain intensity (standardised mean difference (SMD) 0.12, 95% confidence interval (CI) -0.1 to 0.34; n = 319; very low-certainty evidence). The evidence is very uncertain about the effect on adverse events, including constipation (RR 0.71, 95% CI 0.45 to 1.13), drowsiness/somnolence (RR 1.03, 95% CI 0.69 to 1.54), nausea (RR 0.85, 95% CI 0.56 to 1.28), and vomiting (RR 0.66, 95% CI 0.38 to 1.15) (very low-certainty evidence). There were no data available for quality of life or participant preference, however, three studies suggested that treatment acceptability may be similar between groups (low-certainty evidence). CR oxycodone versus CR morphine The majority of the 24 studies comparing CR oxycodone to CR morphine reported either pain intensity (continuous variable), pain relief (dichotomous variable), or both. Pooled analysis indicated that pain intensity may be lower (better) after treatment with CR morphine than CR oxycodone (SMD 0.14, 95% CI 0.01 to 0.27; n = 882 in 7 studies; low-certainty evidence). This SMD is equivalent to a difference of 0.27 points on the Brief Pain Inventory scale (0-10 numerical rating scale), which is not clinically significant. Pooled analyses also suggested that there may be little to no difference in the proportion of participants achieving complete or significant pain relief (RR 1.02, 95% CI 0.95 to 1.10; n = 1249 in 13 studies; low-certainty evidence). The RR for constipation (RR 0.75, 95% CI 0.66 to 0.86) may be lower after treatment with CR oxycodone than after CR morphine. Pooled analyses showed that, for most of the adverse events, the CIs were wide, including no effect as well as potential benefit and harm: drowsiness/somnolence (RR 0.88, 95% CI 0.74 to 1.05), nausea (RR 0.93, 95% CI 0.77 to 1.12), and vomiting (RR 0.81, 95% CI 0.63 to 1.04) (low or very low-certainty evidence). No data were available for quality of life. The evidence is very uncertain about the treatment effects on treatment acceptability and participant preference. Other comparisons The remaining studies either compared oxycodone in various formulations or compared oxycodone to different alternative opioids. None found any clear superiority or inferiority of oxycodone for cancer pain, neither as an analgesic agent nor in terms of adverse event rates and treatment acceptability. The certainty of this evidence base was limited by the high or unclear risk of bias of the studies and by imprecision due to low or very low event rates or participant numbers for many outcomes.
Authors' conclusions: The conclusions have not changed since the previous version of this review (in 2017). We found low-certainty evidence that there may be little to no difference in pain intensity, pain relief and adverse events between oxycodone and other strong opioids including morphine, commonly considered the gold standard strong opioid. Although we identified a benefit for pain relief in favour of CR morphine over CR oxycodone, this was not clinically significant and did not persist following sensitivity analysis and so we do not consider this important. However, we found that constipation and hallucinations occurred less often with CR oxycodone than with CR morphine; but the certainty of this evidence was either very low or the finding did not persist following sensitivity analysis, so these findings should be treated with utmost caution. Our conclusions are consistent with other reviews and suggest that, while the reliability of the evidence base is low, given the absence of important differences within this analysis, it seems unlikely that larger head-to-head studies of oxycodone versus morphine are justified, although well-designed trials comparing oxycodone to other strong analgesics may well be useful. For clinical purposes, oxycodone or morphine can be used as first-line oral opioids for relief of cancer pain in adults.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
MSH: none known.
MIB: none known.
SA: none known.
NB: none known.
JSH: none known. JSH is a Network Associate Editor for the Cochrane MOSS Network. PaPaS CRG is a member of the MOSS Network. JSH had no involvement in the editorial process and/or management of the peer review of this manuscript.
AJP: none known. AJP is a Palliative Care Specialty Trainee (ST5), Leeds Teaching Hospitals NHS Foundation Trust.
YC: none known.
Figures
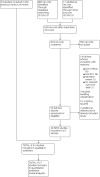































Update of
-
Oxycodone for cancer-related pain.Cochrane Database Syst Rev. 2017 Aug 22;8(8):CD003870. doi: 10.1002/14651858.CD003870.pub6. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Jun 9;6:CD003870. doi: 10.1002/14651858.CD003870.pub7. PMID: 28829910 Free PMC article. Updated.
References
References to studies included in this review
Beaver 1978a {published data only}
-
- Beaver WT, Wallenstein SL, Rogers A, Houde RW. Analgesic studies of codeine and oxycodone in patients with cancer. I. Comparisons of oral with intramuscular codeine and of oral with intramuscular oxycodone. Journal of Pharmacology and Experimental Therapeutics 1978;207(1):92-100. - PubMed
Beaver 1978b {published data only}
-
- Beaver WT, Wallenstein SL, Rogers A, Houde RW. Analgesic studies of codeine and oxycodone in patients with cancer. II. Comparisons of intramuscular oxycodone with intramuscular morphine and codeine. Journal of Pharmacology and Experimental Therapeutics 1978;207(1):101-8. - PubMed
Bruera 1998 {published data only}
-
- Bruera E, Belzile M, Pituskin E, Fainsinger R, Darke A, Harsanyi Z, et al. Randomized, double-blind, cross-over trial comparing safety and efficacy of oral controlled-release oxycodone with controlled-release morphine in patients with cancer pain. Journal of Clinical Oncology 1998;16(10):3222-9. - PubMed
-
- Comerford T, Bruera E. Efficacy of controlled-release oxycodone. Journal of Clinical Oncology 1999;17:738. - PubMed
Cao 2015 {published data only}
-
- Cao Q, Wang LX. The analysis of the two different opioid efficacy in the elderly cancer patients and its safety. Journal of Practical Medical Techniques 2015;22(4):360-1.
Corli 2016 {published data only}
-
- Corli O, Floriani I, Roberto A, Montanari M, Galli F, Greco MT, et al, CERP Study of Pain Group. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV 'real life' trial on the variability of response to opioids. Annals of Oncology 2016;27:1107-15. - PubMed
-
- Corli O, Santucci C, Corsi N, Radrezza S, Galli F, Bosetti C. The burden of opioid adverse events and the influence on cancer patients’ symptomatology. Journal of Pain and Symptom Management 2019;57(5):899-908. - PubMed
-
- NCT01809106. RCT comparing the analgesic efficacy of 4 therapeutic strategies based on 4 different major opioids (fentanyl, oxycodone, buprenorphine vs morphine) in cancer patients with moderate/severe pain, at the moment of starting 3rd step of WHO analgesic ladder. clinicaltrials.gov/show/NCT01809106 (date first received 12 March 2013).
Gabrail 2004 {published data only}
-
- Gabrail NY, Dvergsten C, Ahdieh H. Establishing the dosage equivalency of oxymorphone extended release and oxycodone controlled release in patients with cancer pain: a randomized controlled study. Current Medical Research and Opinion 2004;20(6):911-8. - PubMed
-
- Gabrail NY, Dvergsten C, Ma T, Frailey A, Ahdieh H. Oxymorphone extended-release (ER) provides safe, effective, and rapid analgesia during opioid radiation: results of a randomized, double-blind, crossover, comparative study with oxycodone controlled-release (CR) [abstract]. Proceedings of the American Society of Clinical Oncology 2003:737.
Gao 2012 {published data only}
-
- Gao W, Gu A, Zhu M, Yao L. Oxycodone hydrochloride sustained-release sustained tablets and morphine sulfate sustained-release tablets for advanced malignancy severe pain. Journal of Basic and Clinical Oncology 2012;25(6):524-6.
Hagen 1997 {published data only}
-
- Hagen NA, Babul N. Comparative clinical efficacy and safety of a novel controlled-release oxycodone formulation and controlled-release hydromorphone in the treatment of cancer pain. Cancer 1997;79(7):1428-37. - PubMed
Heiskanen 1997 {published data only}
Imanaka 2013 {published data only}
-
- Imanaka K, Tominaga Y, Etropolski M, Van Hove I, Ohsaka M, Wanibe M, et al. Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain. Current Medical Research and Opinion 2013;29:1399-409. - PubMed
Inoue 2017 {published data only}
-
- Inoue S, Saito Y, Tsuneto S, Aruga E, Ide A, Kakurai Y. A randomized, double-blind study of hydromorphone hydrochloride extended-release tablets versus oxycodone hydrochloride extended-release tablets for cancer pain: efficacy and safety in Japanese cancer patients (EXHEAL: a Phase III study of EXtended-release HydromorphonE for cAncer pain reLief). Journal of Pain Research 2017;10:1953-61. - PMC - PubMed
Inoue 2018 {published data only}
-
- Inoue S, Saito Y, Tsuneto S, Aruga E, Takahashi H, Uemori M. A randomized, double-blind, non-inferiority study of hydromorphone hydrochloride immediate-release tablets versus oxycodone hydrochloride immediate-release powder for cancer pain: efficacy and safety in Japanese cancer patients. Japanese Journal of Cinical Oncology 2018;48(6):542-7. - PMC - PubMed
Kalso 1990 {published data only}
-
- Kalso E, Vainio A, Mattila MJ, Rosenberg PH, Seppala T. Morphine and oxycodone in the management of cancer pain: plasma levels determined by chemical and radioreceptor assays. Pharmacology & Toxicology 1990;67(4):322-8. - PubMed
-
- Kalso E, Vainio A. Morphine and oxycodone hydrochloride in the management of cancer pain. Clinical Pharmacology and Therapeutics 1990;47(5):639-46. - PubMed
Kaplan 1998 {published data only}
-
- Kaplan R, Parris WC, Citron ML, Zhukovsky D, Reder RF, Buckley BJ, et al. Comparison of controlled-release and immediate-release oxycodone tablets in patients with cancer pain. Journal of Clinical Oncology 1998;16(10):3230-7. - PubMed
Lauretti 2003 {published data only}
Lee 2017 {published data only}
-
- NCT02660229. An interventional study for patients with cancer pain to evaluate the efficacy and safety of OxyNorm® compared to morphine sulfate through IV continuous infusion. clinicaltrials.gov/show/NCT02660229 (date received 21 January 2016).
-
- Oh HS, Lee KH, Kang JH, Choi MK, Shim BY, Eum YJ, at al. Efficacy and safety of oxynorm compared to morphine sulfate administering through iv continuous infusion in moderate-severe cancer-related pain.. Supportive Care in Cancer 2017;25 (2 Supplement 1):S90.
Leow 1995 {published data only}
-
- Leow KP, Cramond T, Smith MT. Pharmacokinetics and pharmacodynamics of oxycodone when given intravenously and rectally to adult patients with cancer pain. Anesthesia and Analgesia 1995;80(2):296-302. - PubMed
Li 2013 {published data only}
-
- Li XQ, Lv J, Jin Z. Clinical observation of oxycodone hydrochloride controlled release tablets and morphine sulfate sustained release tablets in the treatment of severe pain in advanced cancer. Journal of Chinese Practical Diagnosis and Therapy 2013;27(12):1216-7.
Liu 2021 {published data only}
Lux 2014 {published data only}
-
- Lux EA, Janecki M, Maritz MA. Clinical evaluation of the first oxycodone once daily prolonged release tablet in moderate to severe chronic pain: a randomized, double-blind, multicenter, cross-over, non-inferiority study to investigate efficacy and safety in comparison with an established oxycodone twice daily prolonged release tablet. Current Medical Research and Opinion 2014;30(11):2365-75. - PubMed
Mercadante 2010 {published data only}
-
- Mercadante S, Tirelli W, David F, Arcara C, Fulfaro F, Casuccio A, et al. Morphine versus oxycodone in pancreatic cancer pain: a randomized controlled study. Clinical Journal of Pain 2010;26(9):794-7. - PubMed
Mucci‐LoRusso 1998 {published data only}
-
- Mucci-LoRusso P, Berman BS, Silberstein PT, Citron ML, Bressler L, Weinstein SM, et al. Controlled-release oxycodone compared with controlled-release morphine in the treatment of cancer pain: a randomized, double-blind, parallel-group study. European Journal of Pain 1998;2(3):239-49. - PubMed
Nosek 2017 {published data only}
-
- Leppert W, Nosek K. Comparison of the quality of life of cancer patients with pain treated with oral controlled-release morphine and oxycodone and transdermal buprenorphine and fentanyl. Current Pharmaceutical Design 2019;25(30):3216-24. - PubMed
-
- Nosek K, Leppert W, Nosek H, Wordliczek J, Onichimowski D. A comparison of oral controlled-release morphine and oxycodone with transdermal formulations of buprenorphine and fentanyl in the treatment of severe pain in cancer patients. Drug Design Development and Therapy 2017;11:2409-19. - PMC - PubMed
Parris 1998 {published data only}
-
- Parris WC, Johnson BW Jr, Croghan MK, Moore MR, Khojasteh A, Reder RF, et al. The use of controlled-release oxycodone for the treatment of chronic cancer pain: a randomized, double-blind study. Journal of Pain and Symptom Management 1998;16(4):205-11. - PubMed
Ren 2012 {published data only}
-
- Ren LP. The pain relief and the quality of life of patients with moderate to severe cancer pain after taking controlled-release tablets of oxycodone hydrochloride and sustained-release tablets of morphine sulfate.. Word Latest Medicine Information 2012;12(9):12-5.
Riley 2015 {published data only}
-
- Riley J, Branford R, Droney J, Gretton S, Sato H, Kennett A, et al. Morphine or oxycodone for cancer-related pain? A randomised, open-label, controlled trial. Journal of Pain and Symptom Management 2015;49(2):161-72. - PubMed
-
- Riley J, Branford R, Droney J, Gretton S, Sato H, Thick M, et al. A randomised controlled trial of oral morphine versus oral oxycodone for the treatment of pain in cancer patients. Palliative Medicine 2012;26(4):386.
Salzman 1999 {published data only}
-
- Salzman RT, Roberts MS, Wild J, Fabian C, Reder RF, Goldenheim PD. Can a controlled-release oral dose form of oxycodone be used as readily as an immediate-release form for the purpose of titrating to stable pain control? Journal of Pain and Symptom Management 1999;18(4):271-9. - PubMed
Song 2015 {published data only}
-
- Song YH. Effectiveness evaluation of oxycodone hydrochloride sustained release tablets and morphine sulfate sustained release tablets for severe cancer pain. China PracMed 2015;10(11):150-1.
Stambaugh 2001 {published data only}
-
- Stambaugh JE, Reder RF, Stambaugh M. Double-blind, randomized, two-period crossover efficacy and pharmacokinetic comparison of immediate-release oxycodone (IR) and controlled-release oxycodone (CR) in cancer patients with pain. Clinical Pharmacology and Therapeutics 1997;61(2):197.
-
- Stambaugh JE, Reder RF, Stambaugh MD, Stambaugh H, Davis M. Double-blind, randomized comparison of the analgesic and pharmacokinetic profiles of controlled- and immediate-release oral oxycodone in cancer pain patients. Journal of Clinical Pharmacology 2001;41(5):500-6. - PubMed
Su 2015 {published data only}
-
- Su J, Zhu Y, Wu W, Luo Y. Observation of curative effects and adverse effects of oxycodone hydrochloride controlled-release tablets and fentanyl transdermal patches on the treatment of moderate or severe cancer pain. Anti-Tumor Pharmacy 2015;5(6):444-8.
Sun 2013 {published data only}
-
- Sun YY, Chen XY, Wang KS. Curative effect analysis of oxycontin and MS contin in the treatment of severe cancerous pain. Cancer Research and Clinic 2013;25(1):36-40.
Tu 2015 {published data only}
-
- Tu XS, Hu LX. Oxycodone hydrochloride sustained release tablets compared with morphine sulfate sustained release tablets in the treatment of advanced moderate to severe cancer pain. Journal of China Prescription Drug 2015;13(10):88-9.
Wang 2008 {published data only}
-
- Wang P, Liu Y, Wang T, Cheng BZ. The observation of curative effect of oxycodone hydrochloride controlled release tablets in management of moderate and severe cancer pain. Modern Oncology 2008;16(11):1983–5.
Xie 2018 {published data only}
-
- Xie XM, Luo LJ, Zhang HL. Clinical efficacy of oxycodone hydrochloride in the treatment of elderly patients with cancer pain. Chinese Community Doctors 2018;34(24):65-7.
Ye 2012 {published data only}
-
- Ye YM, Xiao XX. Analysis of efficacy and adverse reactions of oxycodone hydrochloride controlled release tablet and morphine sulfate sustained-release tablets in the treatment of 83 cases of patients with severe cancer pain. Medical Frontier 2012;2(8):69-70.
Yu 2007 {published data only}
-
- Yu XH, Wu DG, Yang JF, Gao M. Curative effect of oxycodone hydrochloride controlled release tablets in the treatment of cancer pain. Chinese Journal of Hospital Pharmacy 2007;27(9):1277-8.
Yu 2009 {published data only}
-
- Yu H, Liang LS, Wang JF, Liu XD, Zhang GZ, Liu EY. Comparison of the effect of oxycodone and morphine controlled-release tablets in the treatment of visceral cancer pain. Chinese Journal of Current Advances in General Surgery 2009;12(9):769-811.
Yu 2014 {published data only}
-
- Yu S, Shen W, Yu L, Hou Y, Han J, Richards HM. Safety and efficacy of once-daily hydromorphone extended-release versus twice-daily oxycodone hydrochloride controlled-release in Chinese patients with cancer pain: a phase 3, randomized, double-blind, multicenter study. Journal of Pain 2014;15(8):835-44. - PubMed
Zecca 2016 {published data only}
-
- Zecca E, Brunelli C, Bracchi P, Biancofiore G, De Sangro C, Bortolussi R, et al. Comparison of the tolerability profile of controlled-release oral morphine and oxycodone for cancer pain treatment. An open-label randomized controlled trial. Journal of Pain and Symptom Management 2016;52(6):783-94. - PubMed
Zhang 2011 {published data only}
-
- Zhang YY, Han T, Wang Y, Liu L, Wang N, Wang YJ. Effect of the titration of oxycodone controlled-release tablets on moderate pain. Journal of Medical Research 2011;40(4):52–4.
Zhang 2014 {published data only}
-
- Zhang W-Z, Yu W-J, Zhao X-L, He B-X. Pharmacoeconomics evaluation of morphone, MS contin and oxycodone in the treatment of cancer pain. Asian Pacific Journal of Cancer Prevention 2014;15(20):8797-800. - PubMed
Zhang 2016a {published data only}
-
- Zhang XQ. Analgesic effect analysis of three opioids for patients with advanced cancer pain. Medical Frontier 2016;6(6):164-5.
References to studies excluded from this review
Ahmedzai 2012 {published data only}
-
- Ahmedzai SH, Friedemann N, Bar-Sela G, Bjorn B, Leyendecker P, Hopp M. A randomized, double-blind, active-controlled, double-dummy, parallel-group study to determine the safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate/severe, chronic cancer pain. Palliative Medicine 2012;26(1):50-60. - PMC - PubMed
Awerbuch 2011 {published data only}
-
- Awerbuch MS. Should opioids be used for chronic non-cancer pain? Medical Journal of Australia 2011;195(5):264-5. - PubMed
Bekkering 2011 {published data only}
-
- Bekkering GE, Soares-Weiser K, Reid K, Kessels AG, Dahan A, Treede RD, et al. Can morphine still be considered to be the standard for treating chronic pain? A systematic review including pair-wise and network meta-analyses. Current Medical Research and Opinion 2011;27:1477-91. - PubMed
Bell 2006 {published data only}
Borchgrevink 2004 {published data only}
-
- Borchgrevink PC, Klepstad P, Kongsgaard UE, Kaasa S. Use of opioids against severe cancer pain [Bruk av opioider mot sterke kreftrelaterte smerter]. Tidsskrift for Den Norske Laegeforening 2004;124(3):337-8. - PubMed
Caraceni 2011 {published data only}
-
- Caraceni A, Pigni A, Brunelli C. Is oral morphine still the first choice opioid for moderate to severe cancer pain? A systematic review within the European Palliative Care Research Collaborative guidelines project. Palliative Medicine 2011;25:402-9. - PubMed
Carroll 2011 {published data only}
-
- Carroll EMA, Kamboj SK, Conroy L, Tookman A, Williams AC, Jones L, et al. Facial affect processing in patients receiving opioid treatment in palliative care: preferential processing of threat in pain catastrophizers. Journal of Pain and Symptom Management 2011;41(6):975-85. - PubMed
Chary 1994 {published data only}
-
- Chary S, Goughnour BR, Moulin DE, Thorpe WR, Harsanyi Z, Darke AC. The dose-response relationship of controlled-release codeine (Codeine Contin) in chronic cancer pain. Journal of Pain and Symptom Management 1994;9(6):363-71. - PubMed
Chen 2009 {published data only}
-
- Chen HP, Jiang JJ, Li JX. Efficacy and safety of oxycodone-acetaminophen for moderate and severe visceral pain in advanced cancer patients. Chinese Journal of New Drugs 2009;18(10):920-2.
De Conno 1991 {published data only}
-
- De Conno F, Ripamonti C, Sbanotto A, Barletta L, Zecca E, Martini C, et al. A clinical study on the use of codeine, oxycodone, dextropropoxyphene, buprenorphine, and pentazocine in cancer pain. Journal of Pain and Symptom Management 1991;6(7):423-7. - PubMed
Dunlop 2013 {published data only}
-
- Dunlop W, Neufeld K. Quality of life benefits and cost impact of prolonged release oxycodone/naloxone versus prolonged release oxycodone in patients with moderate to severe pain and opioid-induced constipation despite the use of 2 laxatives: a UK cost utility analysis. Value in Health 2013;16(7):A384. - PubMed
Fallon 2011 {published data only}
-
- Fallon MT, Laird BJA. A systematic review of combination step III opioid therapy in cancer pain: an EPCRC opioid guideline project. Palliative Medicine 2011;25(5):597-603. - PubMed
Gao 2020 {published data only}
-
- Gao Y, Zhang B, Li X, Wang D, Sui Y, Li G, et al. Synergistic effect of rosuvastatin with oxycodone hydrochloride sustained-release tablets in the treatment of cancer pain in patients with severe breast cancer and its effect on inflammatory factors. Acta Medica Mediterranea 2020;36(4):2353-7.
Garassino 2010 {published data only}
-
- Garassino MC, Febbraro A, Iorno V, Carbone A, Spagnoletti I, Isa L, et al. Randomized phase ii trial (NCT00637975) evaluating activity and toxicity of fixed dose of oxycodone and increasing dose of pregabalin versus increasing dose of oxycodone and fixed dose of pregabalin for the treatment of oncological neuropathic pain (neuropain-01). Annals of Oncology 2010;21:viii363.
Garassino 2011 {published data only}
-
- Garassino MC, Bianchi A, Febbraro A, Spagnoletti I, Iorno V, Bramati A, et al. Final results of a randomized phase II trial (NCT00637975) evaluating activity and toxicity of fixed-dose oxycodone and increasing dose of pregabalin versus increasing dose of oxycodone and fixed-dose pregabalin for the treatment of oncologic neuropathic pain (NEUROPAIN-01). Journal of Clinical Oncology (ASCO Annual Meeting) 2011;29(15 Suppl 1):9028.
Garassino 2013 {published data only}
-
- Garassino MC, Piva S, Verde N, Spagnoletti I, Iorno V, Carbone C, et al. Randomised phase II trial (NCT00637975) evaluating activity and toxicity of two different escalating strategies for pregabalin and oxycodone combination therapy for neuropathic pain in cancer patients. PlOS One 2013;8(4):e59981. - PMC - PubMed
George 2003 {published data only}
-
- George B, Douard MC, Dubreuil ML. Oxycodone: alternative therapy in the management of chronic cancer pain [L'oxycodone: alternative therapeutique dans la prise en charge de la douleur chronique d'origine cancereuse]. Cahiers d'Anesthesiologie 2003;51:HS7-12.
Guo 2017 {published data only}
-
- Guo J, Lu J, Liu B, Xing L, Xiao J, Wang Z. Effect of hydrochloride oxycodone controlled-release tablets with dose titration for moderate or severe cancer pain [中重度癌痛盐酸羟考酮缓释片治疗 剂量调整临床研究]. Chinese Journal of Cancer Prevention Treatment 2017;24(18):1319-22.
Hanks 2002 {published data only}
-
- Hanks G. Morphine and other opioids in the treatment of cancer pain? International Journal of Cancer 2002;13(Suppl):5.
Hongmei 2013 {published data only}
-
- Hongmei X, Jianwen S. Compound matrine injection in liver carcinoma pain: a clinical study. Journal of Gastroenterology and Hepatology 2013;28:790.
Huang 2015 {published data only}
-
- Huang MQ, Li CF, Kong FL, Gan XQ, Chu XF. Clinical efficacy of oxycodone hydrochloride sustained release tablets in the treatment of pain in advanced cancer. Anhui Medical and Pharmaceutical Journal 2015;19(10):2022-3.
Igarashi 2015 {published data only}
-
- Igarashi T, Abe K, Miura T, Kinoshita H. Oxycodone frequently induced nausea and vomiting in oxycodone-naive patients with hepatic dysfunction. Journal of Palliative Medicine 2015;18(5):399. - PubMed
Katz 2008 {published data only}
-
- Katz MH, Kotabe S. Decreasing use of controlled-release oxycodone - response. Medical Care 2008;46(9):1002. - PubMed
Kim 2015 {published data only}
King 2011 {published data only}
-
- King SJ, Reid C, Forbes K, Hanks G. A systematic review of oxycodone in the management of cancer pain. Palliative Medicine 2011;25:454-70. - PubMed
Koyyalagunta 2012 {published data only}
-
- Koyyalagunta D, Bruera E, Solanki DR, Nouri KH, Burton AW, Toro MP, et al. A systematic review of randomized trials on the effectiveness of opioids for cancer pain. Pain Physician 2012;15(3 Suppl):ES39-58. - PubMed
Kummer 2011 {published data only}
-
- Kummer O, Hammann F, Moser C, Schaller O, Drewe J, Krahenbuhl S. Effect of the inhibition of CYP3A4 or CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. European Journal of Clinical Pharmacology 2011;67(1):63-71. - PubMed
LeBon 2009 {published data only}
-
- LeBon B, Zeppetella G, Higginson IJ. Effectiveness of topical administration of opioids in palliative care: a systematic review. Journal of Pain and Symptom Management 2009;37(5):913-7. - PubMed
Leppert 2011 {published data only}
-
- Leppert W, Ahmedzai SH, Uhl R, Kremers W, Hopp M. Long-term efficacy and safety of the fixed combination oxycodone and naloxone prolonged release (PR) in patients with chronic cancer pain. European Journal of Pain Supplements 2011;5(1):172.
Li 2008 {published data only}
-
- Li RC, He BS. Therapeutic outcome of Tylox for cancer pain in malignant tumor patients with bone metastasis. Chinese Journal of New Drugs 2008;17(18):1619-21.
Li 2010 {published data only}
-
- Li XM, Liu DQ, Wu HY, Yang C, Yang L. Controlled-release oxycodone alone or combined with gabapentin for management of malignant neuropathic pain. Chinese Journal of Cancer Research 2010;22(1):80-6.
Liang 2021 {published data only}
-
- Liang J, Chen L, Yang S, Zhang H, Li L, Chen Z, et al. A 12-hour rapid titration method for cancer pain: a randomized, controlled, open-label study. Annals of Palliative Medicine 2021;10(1):88-96. - PubMed
-
- The feasibility study of sustained-release opioid used for quick analgesia. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TIR-17012925 (date first received 17 October 2017).
Lin 2013 {published data only}
-
- Lin XH, Xu JP, Mai YZ. The efficacy and safety observation of oxycodone retard tablets in the treatment of moderately severe pain. China Medicine and Pharmacy 2013;3(9):78-9.
Ma 2016 {published data only}
Marineo 2012 {published data only}
-
- Marineo G, Iorno V, Gandini C, Moschini V, Smith TJ. Scrambler therapy may relieve chronic neuropathic pain more effectively than guideline-based drug management: results of a pilot, randomized, controlled trial. Journal of Pain and Symptom Management 2012;43(1):87-95. - PubMed
Meng 2008 {published data only}
-
- Meng XL, Tang YL, Zhang JH. Effectiveness observation on oxycodone hydrochloride controlled-release tablets for cancer pain [盐酸羟考酮控释片用于癌性疼痛疗效观察]. Modern Journal of Integrated Traditional Chinese and Western Medicine 2008;17(2):199-200.
Moertel 1974 {published data only}
-
- Moertel CG, Ahmann DL, Taylor WF, Schwartau N. Relief of pain by oral medications. A controlled evaluation of analgesic combinations. Journal of the American Medical Association 1974;229(1):55-9. - PubMed
Moksnes 2012 {published data only}
-
- Moksnes K, Kaasa S, Paulsen Ã, Rosland JH, Spigset O, Dale O. Serum concentrations of opioids when comparing two switching strategies to methadone for cancer pain. European Journal of Clinical Pharmacology 2012;8:1147-56. - PubMed
Mosley 2018 {published data only}
Nadstawek 2008 {published data only}
Nalamachu 2013 {published data only}
-
- Nalamachu S, Rauck R, Dillaha L, Parikh N. Lack of correlation between the dose of fentanyl sublingual spray for breakthrough cancer pain and the dose of around-the-clock opioid for persistent pain. Journal of Pain 2013;1:S74. - PubMed
NCT01859715 {published data only}
-
- NCT01859715. Emergency department (ED) drug interaction in emergency department patients. clinicaltrials.gov/ct2/show/results/NCT01859715 Date first received: 1 May 2013.
NCT01885182 {published data only}
-
- NCT01885182. Targin cancer pain. clinicaltrials.gov/ct2/show/NCT01885182 Date first received: 19 June 2013.
Nunez Olarte 2008 {published data only}
-
- Nunez Olarte JM. Oxycodone and the challenge of neuropathic cancer pain: a review. Oncology 2008;74:83-90. - PubMed
Oosten 2015 {published data only}
-
- Oosten AW, Oldenmenger WH, Mathijssen RH, Van der Rijt CC. A systematic review of prospective studies reporting adverse events of commonly used opioids for cancer-related pain: a call for the use of standardized outcome measures. Journal of Pain 2015;16(10):935-46. - PubMed
Pan 2019 {published data only}
Pang 2009 {published data only}
-
- Pang J, Jie BJ, Li YP. Efficacy of oxycodone-acetominophen by different administrations in controlling moderate to severe cancer pain and break-out pain. Chinese Journal of New Drugs 2009;18(18):1767-70, 1776.
Passik 2014 {published data only}
-
- Passik SD, Narayana A, Yang R. Aberrant drug-related behavior observed during a 12-week open-label extension period of a study involving patients taking chronic opioid therapy for persistent pain and fentanyl buccal tablet or traditional short-acting opioid for breakthrough pain. Pain Medicine 2014;15(8):1365-72. - PubMed
Reid 2006 {published data only}
-
- Reid CM, Martin RM, Sterne JAC, Davies AN, Hanks GW. Oxycodone for cancer-related pain: meta-analysis of randomized controlled trials. Archives of Internal Medicine 2006;166:837-43. - PubMed
Rentz 2009 {published data only}
-
- Rentz AM, Yu R, Muller-Lissner S, Leyendecker P. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. Journal of Medical Economics 2009;12(4):371-83. - PubMed
Riley 2008 {published data only}
-
- Riley J, Eisenberg E, Müller-Schwefe G, Drewes AM, Arendt-Nielsen L. Oxycodone: a review of its use in the management of pain. Current Medical Research and Opinion 2008;24(1):175-92. - PubMed
Shi 2008 {published data only}
-
- Shi L, Xiong HH, Yang L, Yu SY. Analgesic effect of oxycodone-acetaminophen tablets in patients with cervical cancer. Chinese Journal of New Drugs 2008;17:1884-5.
Shi 2018 {published data only}
-
- Shi Z, Lou G, Gu C, Song Z, Shao L, Zhang Y. Efficacy and safety of titration with controlled-release oxycodone versus immediate-release morphine in patients with moderate cancer pain. International Journal of Clinical and Experimental Medicine 2018;11(3):2595-602.
Sima 2010a {published data only}
-
- Sima L, Wu X, Fang W, Li F. Oxycodone/acetaminophen addition to constant opioids in metastatic bone pain: a randomized, double-blinded, controlled, multicenter trial. Journal of Clinical Oncology 2010;28(15 Suppl 1):e19691.
Sima 2010b {published data only}
-
- Sima L, Wu X, Fang W. Oxycodone/acetaminophen combination tablet in patients with metastatic bone pain: a randomized, double-blinded, placebo-controlled trial. Annals of Oncology 2010;21:378.
Sima 2012 {published data only}
-
- Sima L, Fang WX, Wu XM, Li F. Efficacy of oxycodone/paracetamol for patients with bone-cancer pain: a multicenter, randomized, double-blinded, placebo-controlled trial. Journal of Clinical Pharmacy and Therapeutics 2012;37(1):27-31. - PubMed
Stambaugh 1980a {published data only}
-
- Stambaugh JEJ, Tejada F, Trudnowski RJ. Double-blind comparisons of zomepirac and oxycodone with aspirin phenacetin caffeine in cancer pain. Journal of Clinical Pharmacology 1980;20(4):261-70. - PubMed
Stambaugh 1980b {published data only}
-
- Stambaugh JEJ. Analgesic equivalence of Tylox® and Percodan®: double-blind crossover study of patients with pain from malignancy. Current Therapeutic Research, Clinical and Experimental 1980;27(2):302-8.
Stambaugh 1981 {published data only}
-
- Stambaugh JE Jr, Sarajian C. Analgesic efficacy of zomepirac sodium in patients with pain due to cancer. Journal of Clinical Pharmacology 1981;21:501-7. - PubMed
Stambaugh 1985 {published data only}
-
- Stambaugh JEJ, McAdams J. A double-blind comparison of ibuprofen vs. placebo in reducing oxycodone-acetaminophen dosage in chronic cancer pain. Clinical Research 1985;33:458A.
Stambaugh 1987 {published data only}
-
- Stambaugh JE, McAdams J. Comparison of the efficacy and safety of oral xorphanol, acetaminophen/oxycodone and placebo in chronic cancer pain. International Journal of Clinical Pharmacology and Therapeutics 1987;41:229.
Stambaugh 1990 {published data only}
-
- Stambaugh J, Drew J, Johnson J. Comparison of flurbiprofen (Ansaid), oxycodone APAP and placebo in cancer pain, after single and repeat oral doses. Clinical Pharmacology and Therapeutics 1990;47:187.
Stambaugh 1991 {published data only}
-
- Stambaugh JE. Multidose analgesic studies in chronic pain models. Advances in Pain Research and Therapy 1991;18:151-63.
Taeron 2002 {published data only}
-
- Taeron C. Extended release oxycodone. An oral opioid analgesic for cancer patients with chronic pain. Revue De L'Infirmiere 2002;85:47-9. - PubMed
Tanaka 2017 {published data only}
-
- Tanaka R, Ishikawa H, Sato T, Shino M, Matsumoto T, Mori K, et al. Incidence of delirium among patients having cancer injected with different opioids for the first time. American Journal of Hospice & Palliative Medicine 2017;34(6):572-6. - PubMed
Wallace 2013 {published data only}
-
- Wallace E, Twomey M, Victory R, O'Reilly M. Intravesical baclofen, bupivacaine, and oxycodone for the relief of bladder spasm. Journal of Palliative Care 2013;29(1):49-51. - PubMed
Wang 2012 {published data only}
Watanabe 2008 {published data only}
-
- Watanabe S, Pereira J, Tarumi Y, Hanson J, Bruera E. A randomized double-blind crossover comparison of continuous and intermittent subcutaneous administration of opioid for cancer pain. Journal of Palliative Medicine 2008;11(4):570-4. - PubMed
Watanabe 2020 {published data only}
-
- Wanatabe K. Analysis of symptoms relieved in addition to pain after administration of oxycodone or morphine to patients with advanced cancer living at home. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 2020;47(5):797-800.. - PubMed
Wei 2016 {published data only}
-
- Wei YG, Zhou CP. The curative effect observation of acupoint catgut embedding to treat lung cancer pain. Journal of Preventive Medicine of Chinese People's Liberation Army [jie fang jun yu fang yi xue za zhi] 2016;34(Suppl 1):297-8.
Wu 2009 {published data only}
-
- Wu H, Ouyang QC, Liu LP, Wang PH. Safety and efficacy of oxycodone/acetaminophen in treatment of moderate and advanced cancer pain. Chinese Journal of Cancer Prevention and Treatment 2009;18:1432-4.
Wu 2015 {published data only}
-
- Wu H, Chen Y, Shen Q, Cao X, Lu L. Application of controlled-release oxycodone and oral immediate-release morphine in the titration treatment of cancer pain [羟考酮缓释片联合吗啡片在癌痛滴定治疗中的应用]. Anti-Tumor Pharmacy 2015;2:112-5.
Xiong 2008 {published data only}
-
- Xiong J, Liang C, Wu X, Zhao YX, Fu L, Zhou YF. Efficacy and safety of oxycodone-acetaminophen tablet in 142 patients with different types of moderate and advanced cancer pain. Chinese Journal of New Drugs 2008;21:1877-9, 1900.
Xu 2008 {published data only}
-
- Xu LH, Quan SC, Wu LB. Treatment beginning with minute dose oxycontin with advanced stage cancer pain. Modern Journal of Integrated Traditional Chinese and Western Medicine 2008;6:859-60.
Yoshimoto 2018 {published data only}
-
- Yoshimoto T, Ryu E, Tomiyasu S, Hojo M, Kokubun H, Matoba M. Efficacy and safety of oxycodone injection for relieving cancer pain: a study in Japan consisting of two open trials for intravenous and subcutaneous administration. Biological & Pharmaceutical Bulletin 2018;41(6):850-7. - PubMed
Zhu 2019 {published data only}
-
- Zhu M, Liang X, Gu Z, Zhang L, Xie G, Yu S, et al. Multicenter trial of titration of morphine versus oxycodone for cancer pain. International Journal of Clinical and Experimental Medicine 2019;12(6):7317-26.
Zou 2009 {published data only}
-
- Zou J, Wang Q, Liu L. Efficacy of oxycodone-acetaminophen as an adjuvant of opioid analgesics for cancer pain. Chinese Journal of New Drugs 2009;18(10):923-4.
References to studies awaiting assessment
Aurilio 2009 {published data only}
-
- Aurilio C, Sansone P, Pace MC, Passavanti MB, Romano SV, Pota V. Evaluation of efficacy and safety of prolonged-release oxycodone at different dosages for the treatment of severe chronic pain. Pain Practice 2009;9:103.
JapicCTI‐090789/090/091 {unpublished data only}
-
- JapicCTI-090789/090/091. An open-label study of intravenous (i.v.) S-811717 (oxycodone hydrochloride solution for injection) in patients with cancer pain. clinicaltrials.jp/user/showCteDetailE.jsp?japicId=JapicCTI-090789 (date first received 30 June 2009).
JapicCTI‐111388 {unpublished data only}
-
- CTI-111388. TK-641 phase 3 study. clinicaltrials.jp/user/showCteDetailE.jsp?japicId=JapicCTI-111388 (date first received 14 January 2011).
NCT00378937 {unpublished data only}
-
- NCT00378937. Oxycodone or standard pain therapy in treating patients with cancer pain. clinicaltrials.gov/ct2/show/NCT00378937?=NCT00378937&draw=2&rank1 (date first received 21 September 2006).
NCT00726830 {unpublished data only}
-
- NCT00726830. Methadone, morphine, or oxycodone in treating pain in patients with cancer. clinicaltrials.gov/ct2/show/NCT00726830?term=NCT00726830&draw=2&... (date first received 1 August 2008).
NCT01493635 {unpublished data only}
-
- NCT01493635. Two step versus the standard three step approach of the WHO analgesic ladder for cancer pain relief. clinicaltrials.gov/ct2/show/NCT01493635?term=NCT01493635&draw=2&... (date first received 16 December 2011).
NCT03439904 {published data only}
-
- NCT03439904. Individualized pharmaceutical-care in outpatients with cancer pain. clinicaltrials.gov/show/NCT03439904 (date first received 20 February 2018).
Song 2009 {published data only}
-
- Song JF, Zhao YL, Zhou HM, Wang HJ, Li KY. Efficacies of oxycodone and morphine sulfate sustained-release tablets in treating moderate and severe cancer pain. Chinese Journal of New Drugs 2009;18(4):337-9.
Zhang 2016 {published data only}
-
- Zhang Y. Efficacy of oxycotin in the treatment of severe cancer pain by rectal administration. Cancer Nursing 2016;39(6 (Suppl 1)):S73.
References to ongoing studies
2008‐002273‐12 {unpublished data only}
-
- 2008-002273-12. Long term opioid administration in oncologic chronic pain: open label, prospective study on efficacy, safety and pharmacogenetic factors. clinicaltrialsregister.eu/ctr-search/search?query=2008-002273-12 (date first received 23 June 2008).
2009‐013118‐28 {unpublished data only}
-
- 2009-013118-28. The buccal fentanyl in cancer pain management measure [Bukkaalinen fentanyyli syöpäpotilaiden toimenpidekivun hoidossa]. clinicaltrialsregister.eu/ctr-search/search?query=2009-013118-28 (date first received 8 June 2009).
ChiCTR1800014268 {unpublished data only}
-
- ChiCTR1800014268. Effecty [sic] and efficacy of hydrochloride oxycodone controlled-release tablets with dose titration at 12h for cancer pain. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800014268 (date first received 2 January 2018).
ChiCTR1800017461 {unpublished data only}
-
- ChiCTR1800017461. Efficacy and safety of different titration regimens for oxycodone hydrochloride sustained-release tablet in the treatment of cancer pain. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800017461 (date first received 31 July 2018).
ChiCTR1900022566 {unpublished data only}
-
- ChiCTR1900022566. Comparison of oxycodone versus morphine in the treatment of patients with severe cancer pain: a randomized controlled trial. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900022566 (date first received 16 April 2019).
ChiCTR2000037845 {published data only}
-
- ChiCTR2000037845. A multicenter randomized controlled clinical trial of hydromorphone injection PCA versus oxycodone sustained release tablets in the treatment of moderate and severe cancer pain. chictr.org.cn/showprojen.aspx?proj=60437 (date first received 3 September 2020).
ChiCTR2100042972 {published data only}
-
- ChiCTR2100042972. Comparative study of sufentanil self-controlled analgesic pump and oxycodone hydrochloride controlled-release tablets in the treatment of moderate and severe cancer pain. chictr.org.cn/showprojen.aspx?proj=64416 (date first received 2 February 2021).
Elsayem 2010 {unpublished data only}
-
- Elsayem AF, Bain KT, Palmer JL, Bearden J, Fisch M. A randomized comparison of oral methadone as a "first-switch" opioid versus opioid switching between sustained-release morphine and oxycodone for oncology outpatients with pain management problems. Journal of Clinical Oncology 2010;28(15 Suppl 1):TPS324.
IRCT20201202049575N1 {published data only}
-
- IRCT20201202049575N1. Comparison of the effect of morphine and oxycodone in relieving pain in patients with bone metastasis pain. google.com/search?q=IRCT20201202049575N1&rlz=1C1GCEB_enGB999GB1000&a... (date first received 9 December 2020).
Matsouka 2017 {published data only}
NCT00916890 {unpublished data only}
-
- NCT00916890. Chronic administration of opioids in cancer chronic pain: an open prospective study on efficacy, safety and pharmacogenetic factors influence. clinicaltrials.gov/ct2/show/NCT00916890?term=NCT00916890&draw=2&... (date first received 10 June 2009).
NCT01165281 {unpublished data only}
-
- NCT01165281. A safety and efficacy study of JNS024 Extended Release (ER) in Japanese and Korean patients with chronic malignant tumor-related cancer pain. clinicaltrials.gov/ct2/show/NCT01165281?term=NCT01165281&draw=2&... (date first received 19 July 2010).
NCT01675622 {unpublished data only}
-
- NCT01675622. Immediate-release oxycodone capsules study in cancer pain. clinicaltrials.gov/ct2/show/NCT01675622?term=NCT01675622&draw=2&... (date first received 30 August 2012).
NCT02084355 {unpublished data only}
-
- NCT02084355. Study of opioid rotation versus opioid escalation in patients with moderate to severe cancer pain. clinicaltrials.gov/ct2/show/NCT02084355 (date first received 5 March 2014).
NCT03024515 {unpublished data only}
-
- NCT03024515. A pilot randomized open-labelled study comparing a structured titration method of immediate- and sustained-release oxycodone versus opioids titration of investigators' choice in advanced cancer patients in Hong Kong. clinicaltrials.gov/ct2/show/NCT03024515?term=NCT03024515&draw=2&... (date first received 19 January 2017).
NCT03176199 {unpublished data only}
-
- NCT03176199. A study to compare the titration efficacy and safety of control-released oxycodone and immediate-released oxycodone in patients with moderate to severe cancer pain. clinicaltrials.gov/ct2/show/NCT03176199 (date first received 5 June 2017).
NCT04808531 {published data only}
-
- NCT04808531. NanaBis™ an oro-buccal administered delta9-tetrahydrocannabinol (d9-THC) & cannabidiol (CBD) medicine for the management of bone pain from metastatic cancers. clinicaltrials.gov/ct2/show/NCT04808531?term=NCT04808531&draw=2&... (date first received 22 March 2021).
UMIN000011756 {unpublished data only}
-
- UMIN000011756. Randomized study of fentanyl citrate versus oxycodone hydrochloride hydrate in patients with unresectable advanced pancreatic cancer. upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=bro... (date first registered 13 September 2013).
Additional references
Bennett 2008
-
- Bennett MI. What evidence do we have that the WHO analgesic ladder is effective in cancer pain? In: McQuay HJ, Moore R, Kalso E, editors(s). Systematic Reviews in Pain Research; Methodology Refined. Seattle (WA): IASP Press, 2008:303-13.
Breivik 2009
-
- Breivik H, Cherny N, Collett B, De Conno F, Filbet M, Foubert AJ, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Annals of Oncology 2009;20:1420-33. - PubMed
Caraceni 2012
-
- Caraceni A, Hanks GW, Kaasa S, Bennett MI, Brunelli C, Cherny N, et al. Use of opioid analgesics in the treatment of cancer pain: evidence based recommendations from the EAPC. Lancet Oncology 2012;13:e58-68. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- GRADEpro Guideline Development Tool [Software]. McMaster University (developed by Evidence Prime, Inc.), 2015. Available from www.gradepro.org.
Greco 2014
-
- Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, et al. Quality of cancer pain management: an update of a systematic review of under-treatment of patients with cancer. Journal of Clinical Oncology 2014;32(36):4149-54. - PubMed
Gudin 2012
-
- Gudin J. Opioid therapies and cytochrome P450 interactions. Journal of Pain and Symptom Management 2012;44:S4-14. - PubMed
Guo 2018
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Lefebvre 2021
-
- LeLefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Leppert 2010
-
- Leppert W. Role of oxycodone and oxycodone/naloxone in cancer pain management. Pharmacological Report 2010;62:578-91. - PubMed
RevMan 2014 [Computer program]
-
- Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
-
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials.. Annals of Internal Medicine 2010;152(11):726-32. - PubMed
Tong 2018
-
- Tong Z, Li F, Ogawa Y, Watanabe N, Furukawa TA. Quality of randomized controlled trials of new generation antidepressants and antipsychotics identified in the China National Knowledge Infrastructure (CNKI): a literature and telephone interview study. BMC Medical Research Methodology 2018;18:96. - PMC - PubMed
Ventafridda 1987
-
- Ventafridda V, Tamburini M, Caraceni A, De Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer 1987;59:851-6. - PubMed
WHO 1986
-
- World Health Organization (WHO). Cancer pain relief. apps.who.int/iris/bitstream/handle/10665/43944/9241561009_eng.pdf?sequen... (accessed prior to 23 Feb 2022).
WHO 2018
-
- World Health Organization (WHO). WHO Guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents. www.who.int/publications/i/item/9789241550390 (accessed prior to 23 Feb 2022). - PubMed
Wu 2009a
Zhang 2016b
-
- Zhang J, Chen X, Zhu Q, Cui J, Cao L, Su J. Methodological reporting quality of randomized controlled trials: a survey of seven core journals of orthopaedics from mainland China over 5 years following the CONSORT statement. Orthopaedics & Traumatology: Surgery & Research 2016;102:933-8. - PubMed
Zhou 2019
-
- Zhou Q, Yang N, Yang K, Estill J, Chen Y. Pharmacologicaltreatments for generalised anxiety disorder: Comment. Lancet 2019;394:1229. - PubMed
Zhou 2020
Ziegler 2016
-
- Ziegler LE, Mulvey MR, Blenkinsopp A, Petty D, Bennett MI. Opioid prescribing for cancer patients in the last year of life: a longitudinal population cohort study. Pain 2016;157(11):2445-51. - PubMed
References to other published versions of this review
Reid 2010
-
- Reid CM, Davies AN, Hanks GW, Martin RM, Sterne JAC. Oxycodone for cancer-related pain. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No: CD003870. [DOI: 10.1002/14651858.CD003870.pub3] - DOI
Schmidt‐Hansen 2013
Schmidt‐Hansen 2015
-
- Schmidt-Hansen M, Bennett MI, Hilgart J. Oxycodone for cancer pain in adult patients. JAMA 2015;314:1282-3. - PubMed
Schmidt‐Hansen 2018
-
- Schmidt-Hansen M, Bennett MI, Arnold S, Bromham N, Hilgart JS. Efficacy, tolerability and acceptability of oxycodone for cancer-related pain in adults: an updated Cochrane systematic review. BMJ Supportive & Palliative Care 2018;8:117-28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

