Cost-analysis of COVID-19 sample collection, diagnosis, and contact tracing in low resource setting: The case of Addis Ababa, Ethiopia
- PMID: 35679290
- PMCID: PMC9182302
- DOI: 10.1371/journal.pone.0269458
Cost-analysis of COVID-19 sample collection, diagnosis, and contact tracing in low resource setting: The case of Addis Ababa, Ethiopia
Abstract
Background: Ethiopia has been responding to the COVID-19 pandemic through a combination of interventions, including non-pharmaceutical interventions, quarantine, testing, isolation, contact tracing, and clinical management. Estimating the resources consumed for COVID-19 prevention and control could inform efficient decision-making for epidemic/pandemic-prone diseases in the future. This study aims to estimate the unit cost of COVID-19 sample collection, laboratory diagnosis, and contact tracing in Addis Ababa, Ethiopia.
Methods: Primary and secondary data were collected to estimate the costs of COVID-19 sample collection, diagnosis, and contact tracing. A healthcare system perspective was used. We used a combination of micro-costing (bottom-up) and top-down approaches to estimate resources consumed and the unit costs of the interventions. We used available cost and outcome data between May and December 2020. The costs were classified into capital and recurrent inputs to estimate unit and total costs. We identified the cost drivers of the interventions. We reported the cost for the following outcome measures: (1) cost per sample collected, (2) cost per laboratory diagnosis, (3) cost per sample collected and laboratory diagnosis, (4) cost per contact traced, and (5) cost per COVID-19 positive test identified. We conducted one-way sensitivity analysis by varying the input parameters. All costs were reported in US dollars (USD).
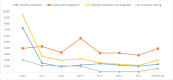
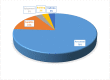
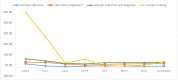
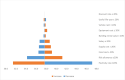
Results: The unit cost per sample collected was USD 1.33. The unit cost of tracing a contact of an index case was USD 0.66. The unit cost of COVID-19 diagnosis, excluding the cost for sample collection was USD 3.91. The unit cost of sample collection per COVID-19 positive individual was USD 11.63. The unit cost for COVID-19 positive test through contact tracing was USD 54.00. The unit cost COVID-19 DNA PCR diagnosis for identifying COVID-19 positive individuals, excluding the sample collection and transport cost, was USD 37.70. The cost per COVID-19 positive case identified was USD 49.33 including both sample collection and laboratory diagnosis costs. Among the cost drivers, personnel cost (salary and food cost) takes the highest share for all interventions, ranging from 51-76% of the total cost.
Conclusion: The costs of sample collection, diagnosis, and contact tracing for COVID-19 were high given the low per capita health expenditure in Ethiopia and other low-income settings. Since the personnel cost accounts for the highest cost, decision-makers should focus on minimizing this cost when faced with pandemic-prone diseases by strengthening the health system and using digital platforms. The findings of this study can help decision-makers prioritize and allocate resources for effective public health emergency response.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Testing for SARS-CoV-2 in resource-limited settings: A cost analysis study of diagnostic tests using different Ag-RDTs and RT-PCR technologies in Mozambique.PLOS Glob Public Health. 2023 Jun 13;3(6):e0001999. doi: 10.1371/journal.pgph.0001999. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 37310935 Free PMC article.
-
Hospitalization costs for COVID-19 in Ethiopia: Empirical data and analysis from Addis Ababa's largest dedicated treatment center.PLoS One. 2022 Jan 21;17(1):e0260930. doi: 10.1371/journal.pone.0260930. eCollection 2022. PLoS One. 2022. PMID: 35061674 Free PMC article.
-
Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial.Trials. 2021 Jan 8;22(1):39. doi: 10.1186/s13063-020-04982-z. Trials. 2021. PMID: 33419461 Free PMC article.
-
A Systematic Review of the Costs Relating to Non-pharmaceutical Interventions Against Infectious Disease Outbreaks.Appl Health Econ Health Policy. 2021 Sep;19(5):673-697. doi: 10.1007/s40258-021-00659-z. Epub 2021 Jun 11. Appl Health Econ Health Policy. 2021. PMID: 34114184 Free PMC article.
-
Applications of digital technology in COVID-19 pandemic planning and response.Lancet Digit Health. 2020 Aug;2(8):e435-e440. doi: 10.1016/S2589-7500(20)30142-4. Epub 2020 Jun 29. Lancet Digit Health. 2020. PMID: 32835201 Free PMC article. Review.
Cited by
-
From Emergence to Endemicity: A Comprehensive Review of COVID-19.Cureus. 2023 Oct 31;15(10):e48046. doi: 10.7759/cureus.48046. eCollection 2023 Oct. Cureus. 2023. PMID: 37916248 Free PMC article. Review.
-
Testing for SARS-CoV-2 in resource-limited settings: A cost analysis study of diagnostic tests using different Ag-RDTs and RT-PCR technologies in Mozambique.PLOS Glob Public Health. 2023 Jun 13;3(6):e0001999. doi: 10.1371/journal.pgph.0001999. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 37310935 Free PMC article.
-
Value for money of medicine sampling and quality testing: evidence from Indonesia.BMJ Glob Health. 2024 Sep 23;9(9):e015402. doi: 10.1136/bmjgh-2024-015402. BMJ Glob Health. 2024. PMID: 39313254 Free PMC article.
-
Community Wastewater-Based Surveillance Can Be a Cost-Effective Approach to Track COVID-19 Outbreak in Low-Resource Settings: Feasibility Assessment for Ethiopia Context.Int J Environ Res Public Health. 2022 Jul 12;19(14):8515. doi: 10.3390/ijerph19148515. Int J Environ Res Public Health. 2022. PMID: 35886369 Free PMC article.
-
Using an app for COVID-19 contact tracing costs less per person traced than manual tracing: microcosting analysis of a randomised trial in Cameroon.BMJ Public Health. 2025 Jan 21;2(Suppl 1):e001064. doi: 10.1136/bmjph-2024-001064. eCollection 2024 Jul. BMJ Public Health. 2025. PMID: 40017704 Free PMC article.
References
-
- Global Surveillance for human infection with coronavirus disease (COVID-19). Interim Guid [Internet]. (February):27–9. Available from: https://www.who.int/publications-detail/global-surveillance-for-human-in... [Internet]. 2020.
-
- www.worldometers. worldometers: COVID-19 CORONAVIRUS PANDEMIC Cases, Deaths and Recovered Report. 2021;263.
-
- Overview of public health and social measures in the context of COVID-19. World Heal Organ 2020. 2020;(May):1–8. [Internet].
-
- World Health Organization. Contact tracing in the context of COVID-19. Interim guidance. Pediatr i Med Rodz. 2020;16(1):33–9.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

