Identifying new drugs associated with pulmonary arterial hypertension: A WHO pharmacovigilance database disproportionality analysis
- PMID: 35679331
- PMCID: PMC9795981
- DOI: 10.1111/bcp.15436
Identifying new drugs associated with pulmonary arterial hypertension: A WHO pharmacovigilance database disproportionality analysis
Abstract
Since the 1960s, several drugs have been linked to the onset or aggravation of pulmonary arterial hypertension (PAH): dasatinib, some amphetamine-like appetite suppressants (aminorex, fenfluramine, dexfenfluramine, benfluorex) and recreational drugs (methamphetamine). Moreover, in numerous cases, the implication of other drugs with PAH have been suggested, but the precise identification of iatrogenic aetiologies of PAH is challenging given the scarcity of this disease and the potential long latency period between drug intake and PAH onset. In this context, we used the World Health Organization's pharmacovigilance database, VigiBase, to generate new hypotheses about drug associated PAH.
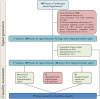
Methods: We used VigiBase, the largest pharmacovigilance database worldwide to generate disproportionality signals through the Bayesian neural network method. All disproportionality signals were further independently reviewed by experts in pulmonary arterial hypertension, pharmacovigilance and vascular pharmacology and their plausibility ranked according to World Health Organization causality categories.
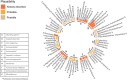
Results: We included 2184 idiopathic PAH cases, yielding a total of 93 disproportionality signals. Among them, 25 signals were considered very likely, 15 probable, 28 possible and 25 unlikely. Notably, we identified 4 new protein kinases inhibitors (lapatinib, lorlatinib, ponatinib and ruxolitinib), 1 angiogenesis inhibitor (bevacizumab), and several chemotherapeutics (etoposide, trastuzumab), antimetabolites (cytarabine, fludarabine, fluorouracil, gemcitabine) and immunosuppressants (leflunomide, thalidomide, ciclosporin).
Conclusion: Such signals represent plausible adverse drug reactions considering the knowledge of iatrogenic PAH, the drugs' biological and pharmacological activity and the characteristics of the reported case. Although confirmatory studies need to be performed, the signals identified may help clinicians envisage an iatrogenic aetiology when faced with a patient who develops PAH.
Keywords: drug safety; pharmacovigilance; pulmonary arterial hypertension; respiratory medicine; vascular disease.
© 2022 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
Dr Roustit reports grants from United Therapeutic outside of the submitted work. Dr Montani reports grants and personal fees from Actelion, grants and personal fees from Bayer, personal fees from GSK, personal fees from Pfizer, grants, personal fees and nonfinancial support from MSD, personal fees from Chiesi, personal fees from Boerhinger, nonfinancial support from Acceleron, and personal fees from Incyte Biosciences France, outside the submitted work. Dr Humbert reports personal fees from Acceleron, grants and personal fees from Actelion, grants and personal fees from Bayer, personal fees from GSK, personal fees from Merck, personal fees from Novartis, personal fees from AstraZeneca, and personal fees from Sanofi, outside the submitted work. Dr Chaumais reports personal fees from Bayer outside the submitted work. Dr Guignabert report grants from Acceleron, Janssen and Merck outside from the submitted work and personal fees Merck. Dr Khouri, Cracowski, Hlavaty have nothing to disclose.
Figures
References
-
- Greiser E. Epidemiologic studies on the relation between use of appetite depressants and primary vascular pulmonary hypertension. Internist. 1973;14(9):437‐442. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

