Heart Failure With Preserved Ejection Fraction: Heterogeneous Syndrome, Diverse Preclinical Models
- PMID: 35679364
- PMCID: PMC10035274
- DOI: 10.1161/CIRCRESAHA.122.320257
Heart Failure With Preserved Ejection Fraction: Heterogeneous Syndrome, Diverse Preclinical Models
Erratum in
-
Correction to: Heart Failure with Preserved Ejection Fraction: Heterogeneous Syndrome, Diverse Preclinical Models.Circ Res. 2022 Aug 5;131(4):e100. doi: 10.1161/RES.0000000000000564. Epub 2022 Aug 4. Circ Res. 2022. PMID: 35926011 No abstract available.
Abstract
Heart failure with preserved ejection fraction (HFpEF) represents one of the greatest challenges facing cardiovascular medicine today. Despite being the most common form of heart failure worldwide, there has been limited success in developing therapeutics for this syndrome. This is largely due to our incomplete understanding of the biology driving its systemic pathophysiology and the heterogeneity of clinical phenotypes, which are increasingly being recognized as distinct HFpEF phenogroups. Development of efficacious therapeutics fundamentally relies on robust preclinical models that not only faithfully recapitulate key features of the clinical syndrome but also enable rigorous investigation of putative mechanisms of disease in the context of clinically relevant phenotypes. In this review, we propose a preclinical research strategy that is conceptually grounded in model diversification and aims to better align with our evolving understanding of the heterogeneity of clinical HFpEF. Although heterogeneity is often viewed as a major obstacle in preclinical HFpEF research, we challenge this notion and argue that embracing it may be the key to demystifying its pathobiology. Here, we first provide an overarching guideline for developing HFpEF models through a stepwise approach of comprehensive cardiac and extra-cardiac phenotyping. We then present an overview of currently available models, focused on the 3 leading phenogroups, which are primarily based on aging, cardiometabolic stress, and chronic hypertension. We discuss how well these models reflect their clinically relevant phenogroup and highlight some of the more recent mechanistic insights they are providing into the complex pathophysiology underlying HFpEF.
Keywords: aging; animal models; atrial fibrillation; blood pressure; edema; heart failure; obesity.
Conflict of interest statement
Disclosures
Dr. Hill is a coinventor on a patent application (PCT/ US/2017/037019) that was filed in June 2017 (provisional application filed in June 2016). The patent relates to the diet used for modeling HFpEF. The other authors report no conflicts.
Figures
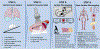


References
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 - DOI - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

