Comparative effectiveness of ZUMA-5 (axi-cel) vs SCHOLAR-5 external control in relapsed/refractory follicular lymphoma
- PMID: 35679476
- PMCID: PMC9412012
- DOI: 10.1182/blood.2021014375
Comparative effectiveness of ZUMA-5 (axi-cel) vs SCHOLAR-5 external control in relapsed/refractory follicular lymphoma
Abstract
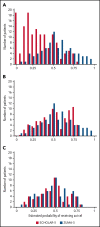
In the pivotal ZUMA-5 trial, axicabtagene ciloleucel (axi-cel; an autologous anti-CD19 chimeric antigen receptor T-cell therapy) demonstrated high rates of durable response in relapsed/refractory (r/r) follicular lymphoma (FL) patients. Here, outcomes from ZUMA-5 are compared with the international SCHOLAR-5 cohort, which applied key ZUMA-5 trial eligibility criteria simulating randomized controlled trial conditions. SCHOLAR-5 data were extracted from institutions in 5 countries, and from 1 historical clinical trial, for r/r FL patients who initiated a third or higher line of therapy after July 2014. Patient characteristics were balanced through propensity scoring on prespecified prognostic factors using standardized mortality ratio (SMR) weighting. Time-to-event outcomes were evaluated using weighted Kaplan-Meier analysis. Overall response rate (ORR) and complete response (CR) rate were compared using weighted odds ratios. The 143 ScHOLAR-5 patients reduced to an effective sample of 85 patients after SMR weighting vs 86 patients in ZUMA-5. Median follow-up time was 25.4 and 23.3 months for SCHOLAR-5 and ZUMA-5. Median overall survival (OS) and progression-free survival (PFS) in SCHOLAR-5 were 59.8 months and 12.7 months and not reached in ZUMA-5. Hazard ratios for OS and PFS were 0.42 (95% confidence interval [CI], 0.21-0.83) and 0.30 (95% CI, 0.18-0.49). The ORR and CR rate were 49.9% and 29.9% in SCHOLAR-5 and 94.2% and 79.1% in ZUMA-5, for odds ratios of 16.2 (95% CI, 5.6-46.9) and 8.9 (95% CI, 4.3-18.3). Compared with available therapies, axi-cel demonstrated an improvement in meaningful clinical endpoints, suggesting axi-cel addresses an important unmet need for r/r FL patients. This trial was registered at www.clinicaltrials.gov as #NCT03105336.
© 2022 by The American Society of Hematology.
Figures


Comment in
-
CAR-T for follicular lymphoma: are we good to go?Blood. 2022 Aug 25;140(8):799-800. doi: 10.1182/blood.2022017279. Blood. 2022. PMID: 36006675 No abstract available.
References
-
- Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90(9):790-795. https://10.1002/ajh.24086. - PubMed
-
- Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459. - PubMed
-
- Pehlivan KC, Duncan BB, Lee DW. CAR-T cell therapy for acute lymphoblastic leukemia: transforming the treatment of relapsed and refractory disease. Curr Hematol Malig Rep. 2018;13(5):396-406. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

