New Insights into Clinical and Mechanistic Heterogeneity of the Acute Respiratory Distress Syndrome: Summary of the Aspen Lung Conference 2021
- PMID: 35679511
- PMCID: PMC9447141
- DOI: 10.1165/rcmb.2022-0089WS
New Insights into Clinical and Mechanistic Heterogeneity of the Acute Respiratory Distress Syndrome: Summary of the Aspen Lung Conference 2021
Abstract
Clinical and molecular heterogeneity are common features of human disease. Understanding the basis for heterogeneity has led to major advances in therapy for many cancers and pulmonary diseases such as cystic fibrosis and asthma. Although heterogeneity of risk factors, disease severity, and outcomes in survivors are common features of the acute respiratory distress syndrome (ARDS), many challenges exist in understanding the clinical and molecular basis for disease heterogeneity and using heterogeneity to tailor therapy for individual patients. This report summarizes the proceedings of the 2021 Aspen Lung Conference, which was organized to review key issues related to understanding clinical and molecular heterogeneity in ARDS. The goals were to review new information about ARDS phenotypes, to explore multicellular and multisystem mechanisms responsible for heterogeneity, and to review how best to account for clinical and molecular heterogeneity in clinical trial design and assessment of outcomes. The report concludes with recommendations for future research to understand the clinical and basic mechanisms underlying heterogeneity in ARDS to advance the development of new treatments for this life-threatening critical illness.
Keywords: ARDS; biological mechanisms; clinical trials; critical care.
Figures

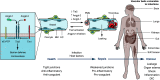


References
-
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin definition. JAMA . 2012;307:2526–2533. - PubMed
-
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet . 1967;2:319–323.
-
- Petty TL, Reiss OK, Paul GW, Silvers GW, Elkins ND. Characteristics of pulmonary surfactant in adult respiratory distress syndrome associated with trauma and shock. Am Rev Respir Dis . 1977;115:531–536. - PubMed
-
- Petty TL, Silvers GW, Paul GW, Stanford RE. Abnormalities in lung elastic properties and surfactant function in adult respiratory distress syndrome. Chest . 1979;75:571–574. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL132232/HL/NHLBI NIH HHS/United States
- R01 HL126176/HL/NHLBI NIH HHS/United States
- P01 HL066299/HL/NHLBI NIH HHS/United States
- 5R01HL14254902/HL/NHLBI NIH HHS/United States
- R01 HL140182/HL/NHLBI NIH HHS/United States
- R01 HL147920/HL/NHLBI NIH HHS/United States
- HL103836/HL/NHLBI NIH HHS/United States
- K24 HL155804/HL/NHLBI NIH HHS/United States
- R01 HL148069/HL/NHLBI NIH HHS/United States
- R41 HL126456/HL/NHLBI NIH HHS/United States
- R01 AG049493/AG/NIA NIH HHS/United States
- R01 GM125095/GM/NIGMS NIH HHS/United States
- R01 HL135849/HL/NHLBI NIH HHS/United States
- R13 HL152479/HL/NHLBI NIH HHS/United States
- R01 HL143896/HL/NHLBI NIH HHS/United States
- R01 HL137006/HL/NHLBI NIH HHS/United States
- RO1HL132232/HL/NHLBI NIH HHS/United States
- K24HL141526/HL/NHLBI NIH HHS/United States
- U01 HL123009/HL/NHLBI NIH HHS/United States
- HL147920/HL/NHLBI NIH HHS/United States
- K24 HL141526/HL/NHLBI NIH HHS/United States
- R01 LM010685/LM/NLM NIH HHS/United States
- HL131608/HL/NHLBI NIH HHS/United States
- R01 HL158906/HL/NHLBI NIH HHS/United States
- R35 HL140026/HL/NHLBI NIH HHS/United States
- R01 HL137915/HL/NHLBI NIH HHS/United States
- HL148069/HL/NHLBI NIH HHS/United States
- R35 HL139424/HL/NHLBI NIH HHS/United States
- HL126176/HL/NHLBI NIH HHS/United States
- R35 HL161241/HL/NHLBI NIH HHS/United States
- HL143896/HL/NHLBI NIH HHS/United States
- K24 HL103836/HL/NHLBI NIH HHS/United States
- R01 HL134828/HL/NHLBI NIH HHS/United States
- 1R01 HL140595/HL/NHLBI NIH HHS/United States
- R01 HL140595/HL/NHLBI NIH HHS/United States
- HL135849/HL/NHLBI NIH HHS/United States
- R01 AI160167/AI/NIAID NIH HHS/United States
- 1UO1HL123009/HL/NHLBI NIH HHS/United States
- R42 HL126456/HL/NHLBI NIH HHS/United States
- HL137006/HL/NHLBI NIH HHS/United States
- HL155804/HL/NHLBI NIH HHS/United States
- HL140026/HL/NHLBI NIH HHS/United States
- HL66299/HL/NHLBI NIH HHS/United States
- HL140182/HL/NHLBI NIH HHS/United States
- HL137915/HL/NHLBI NIH HHS/United States
- R01 HL131608/HL/NHLBI NIH HHS/United States
- HL126456/HL/NHLBI NIH HHS/United States
- GM12509/GM/NIGMS NIH HHS/United States
- HL134828/HL/NHLBI NIH HHS/United States
- I01 BX003471/BX/BLRD VA/United States
- R01 AI165919/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

