Patient-reported systemic symptoms in women with silicone breast implants: a descriptive cohort study
- PMID: 35680258
- PMCID: PMC9185500
- DOI: 10.1136/bmjopen-2021-057159
Patient-reported systemic symptoms in women with silicone breast implants: a descriptive cohort study
Abstract
Objective: An unknown portion of women with silicone breast implants (SBI) report development of systemic symptoms, recently named as 'breast implant illness (BII)'. We aim to describe the symptoms and characteristics of women with SBI reporting these systemic symptoms and compare the clinical course of women who chose to keep their implants, to women who had their implants removed.
Design: Observational cohort study.
Setting: Specialised BII out-patient clinic at Amsterdam UMC, the Netherlands, from 2011 to 2020.
Participants: All women presenting to the BII clinic with SBI and systemic symptoms.
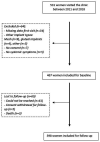
Results: 467 women were included for baseline analyses and 398 women for follow-up. Most frequently reported systemic symptoms at baseline included fatigue (88%), arthralgia (71%), morning stiffness (59%), myalgia (48%), cognitive impairment (33%), peripheral neurological symptoms (30%) and lymphadenopathy (22%). Furthermore, 56% reported pre-existing allergies at baseline and positive antinuclear antibodies were observed in 23%. At follow-up with a median of 3.3 years (IQR 2-4), 152 women had their implants removed on clinical grounds. Symptoms improved significantly in 65 women (43%), improved moderately in 37 women (24%), did not change in 37 women (24%) and deteriorated in 13 women (9%). Women who underwent explantation showed more improvement of their systemic symptoms compared with women who did not (OR 2.9, 95% CI 1.3 to 6.2). Additionally, women who underwent explantation within 10 years after implantation improved significantly better than women who got the implants removed after 10 years (p=0.007). Lastly, local symptoms decreased from 75% to 34% after implant removal (p<0.0001).
Conclusion: Most women with SBI who developed systemic symptoms experienced improvement after explantation, especially when removed within 10 years after implantation. Early recognition of the pattern of systemic symptoms in women with SBI is important and implant removal should be considered.
Keywords: breast surgery; plastic & reconstructive surgery; rheumatology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical