Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine
- PMID: 35680872
- PMCID: PMC9184525
- DOI: 10.1038/s41467-022-30884-6
Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine
Abstract
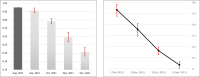
The duration of protection of the third (booster) dose of the BioNTech/Pfizer BNT162b2 mRNA Coronavirus Disease 2019 vaccine has been the subject of recent investigations, as global discussions around the necessity and effectiveness of a fourth dose are already underway. By conducting a retrospective study implementing a test-negative case-control design, analyzing 546,924 PCR tests performed throughout January 2022 by 389,265 persons who received at least two doses, we find that the effectiveness in each month-since-vaccination decreases significantly. Compared to those vaccinated five months prior to the outcome period, on August 2021, relative protection against infection waned from 53.4% a month after vaccination to 16.5% three months after vaccination. These results suggest that there is a significant waning of vaccine effectiveness against the Omicron variant of the third dose of the BNT162b2 vaccine within a few months after administration. Additional information could assist to comprehensively estimate the effectiveness of the three-dose-strategy.
© 2022. The Author(s).
Conflict of interest statement
Y.M. received lecture fees and a quality grant from Pfizer. All other authors declare they have no competing interests.
Figures

References
-
- Haas EJ, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

