Norepinephrine potentiates and serotonin depresses visual cortical responses by transforming eligibility traces
- PMID: 35680879
- PMCID: PMC9184610
- DOI: 10.1038/s41467-022-30827-1
Norepinephrine potentiates and serotonin depresses visual cortical responses by transforming eligibility traces
Erratum in
-
Author Correction: Norepinephrine potentiates and serotonin depresses visual cortical responses by transforming eligibility traces.Nat Commun. 2022 Jul 12;13(1):4034. doi: 10.1038/s41467-022-31777-4. Nat Commun. 2022. PMID: 35821244 Free PMC article. No abstract available.
Abstract
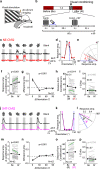
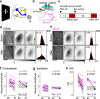
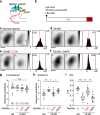
Reinforcement allows organisms to learn which stimuli predict subsequent biological relevance. Hebbian mechanisms of synaptic plasticity are insufficient to account for reinforced learning because neuromodulators signaling biological relevance are delayed with respect to the neural activity associated with the stimulus. A theoretical solution is the concept of eligibility traces (eTraces), silent synaptic processes elicited by activity which upon arrival of a neuromodulator are converted into a lasting change in synaptic strength. Previously we demonstrated in visual cortical slices the Hebbian induction of eTraces and their conversion into LTP and LTD by the retroactive action of norepinephrine and serotonin Here we show in vivo in mouse V1 that the induction of eTraces and their conversion to LTP/D by norepinephrine and serotonin respectively potentiates and depresses visual responses. We also show that the integrity of this process is crucial for ocular dominance plasticity, a canonical model of experience-dependent plasticity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures



References
-
- Mackintosh NJ. Blocking of conditioned suppression: role of the first compound trial. J. Exp. Psychol.: Anim. Behav. Process. 1975;1:335–345. - PubMed

