Elevated plasma level of the glycolysis byproduct methylglyoxal on admission is an independent biomarker of mortality in ICU COVID-19 patients
- PMID: 35680931
- PMCID: PMC9178541
- DOI: 10.1038/s41598-022-12751-y
Elevated plasma level of the glycolysis byproduct methylglyoxal on admission is an independent biomarker of mortality in ICU COVID-19 patients
Abstract
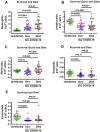
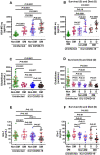
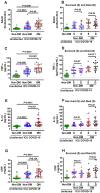
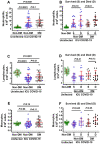
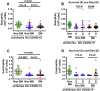
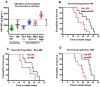
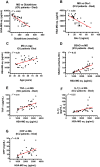
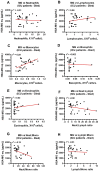
Biomarkers to identify ICU COVID-19 patients at high risk for mortality are urgently needed for therapeutic care and management. Here we found plasma levels of the glycolysis byproduct methylglyoxal (MG) were 4.4-fold higher in ICU patients upon admission that later died (n = 33), and 1.7-fold higher in ICU patients that survived (n = 32),compared to uninfected controls (n = 30). The increased MG in patients that died correlated inversely with the levels of the MG-degrading enzyme glyoxalase-1 (r2 = - 0.50), and its co-factor glutathione (r2 = - 0.63), and positively with monocytes (r2 = 0.29). The inflammation markers, SSAO (r2 = 0.52), TNF-α (r2 = 0.41), IL-1β (r2 = 0.25), CRP (r2 = 0.26) also correlated positively with MG. Logistic regression analysis provides evidence of a significant relationship between the elevated MG upon admission into ICU and death (P < 0.0001), with 42% of the death variability explained. From these data we conclude that elevated plasma MG on admission is a novel independent biomarker that predicts mortality in ICU COVID-19 patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Johns Hopkins University and Medicine (2021). Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE). https://coronavirus.jhu.edu/map.html (2021). Accessed 20 May 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

