Effects of oral treatment with chondroitin sulfate and glucosamine in an experimental model of metacarpophalangeal osteoarthritis in horses
- PMID: 35681208
- PMCID: PMC9178899
- DOI: 10.1186/s12917-022-03323-3
Effects of oral treatment with chondroitin sulfate and glucosamine in an experimental model of metacarpophalangeal osteoarthritis in horses
Abstract
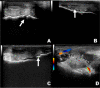
Background: Combined chondroitin sulfate (CS) and glucosamine (GlcN) has been widely used in oral formulations to prevent and treat osteoarthritis. CS is effective for controlling pain in osteoarthritic patients, whereas GlcN can stimulate glycosaminoglycan synthesis, thus reducing extracellular matrix degradation. Although several studies have been published on this topic, the effectiveness of treatment with oral CS and GlcN remains uncertain. The objective of this study was to analyze the progression of experimentally induced osteoarthritis in horses and verify the effectiveness of an oral compound based on CS and GlcN to treat and/or modulate this disease. The study analyzed the metacarpophalangeal joint of the left thoracic limb of 16 horses divided into two groups, with eight horses treated with CS and GlcN in the treated group (GT) and eight untreated horses in the control group (GC). Chondral lesions were induced through arthroscopy, which was defined as time-point zero (T0). Physical, ultrasonographic, and radiographic examinations and synovial fluid biomarkers measurements were performed on days 0, 30, 60, 90, and 120. At the end of the experiment (T4), arthroscopy was performed again to macroscopically evaluate the joints and collect material for microscopic analysis.
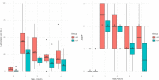
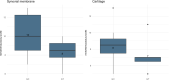
Results: Significant differences were observed between groups in some evaluated parameters, such as visual lameness assessment, synovial concentrations of prostaglandin E2, and ultrasound examination. However, the GT still presented slightly improved results for joint flexion angle, analysis of lameness using sensors, and histopathological analysis of chondral repair tissue, however, without the statistical significance (p>0.05).
Conclusions: The treatment was considered effective in the clinical modulation of experimental osteoarthritis, with improvement of some parameters in the GT. However, this type of treatment may not be entirely effective to change the catabolic process in articular cartilage and the progressive induced chondral damage.
Keywords: Equine; Glycosaminoglycan; Joint; Lameness; Osteoarthritis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Monteiro SO, Bettencourt EV, Lepage OM. Biologic strategies for intra-articular treatment and cartilage repair. J Equine Vet Sci. 2015;35(3):175–190. doi: 10.1016/j.jevs.2015.01.006. - DOI
-
- Elmesiry AM, Seleim MA, Mansour AA, Hill DC. Pentosan polysulfate as a disease modifier of cartilage degeneration in experimental osteoarthritis. J Arthritis. 2016;5(199):2.
-
- McIlwraith CW, Kawcak CE, Frisbie DD, Little CB, Clegg PD, Peffers MJ, et al. Biomarkers for equine joint injury and osteoarthritis. J Orthop Res. 2018;36(3):823–831. - PubMed
-
- Te Moller N. Development of an equine carpal groove model to study early changes in osteoarthritis-a pilot study. Osteoarthr Cartil. 2018;26(1):S132–S133. doi: 10.1016/j.joca.2018.02.288. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

