CXCR4 knockdown enhances sensitivity of paclitaxel via the PI3K/Akt/mTOR pathway in ovarian carcinoma
- PMID: 35681259
- PMCID: PMC9217704
- DOI: 10.18632/aging.203241
CXCR4 knockdown enhances sensitivity of paclitaxel via the PI3K/Akt/mTOR pathway in ovarian carcinoma
Erratum in
-
Correction for: CXCR4 knockdown enhances sensitivity of paclitaxel via the PI3K/Akt/mTOR pathway in ovarian carcinoma.Aging (Albany NY). 2022 Nov 15;14(21):3891-3892. doi: 10.18632/aging.204735. Epub 2022 Nov 15. Aging (Albany NY). 2022. PMID: 36377208 Free PMC article. No abstract available.
Abstract
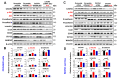
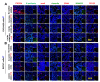
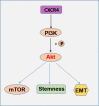
Epithelial ovarian cancer (EOC) is the deadliest gynecological malignancy. EOC control remains difficult, and EOC patients show poor prognosis regarding metastasis and chemotherapy resistance. The aim of this study was to estimate the effect of CXCR4 knockdown-mediated reduction of cancer stem cells (CSCs) and epithelial-mesenchymal transition (EMT) stemness and enhancement of chemotherapy sensitivity in EOC. Mechanisms contributing to these effects were also explored. Our data showed distinct contribution of CXCR4 overexpression by dependent PI3K/Akt/mTOR signaling pathway in EOC development. CXCR4 knockdown resulted in a reduction in CSCs and EMT formation and enhancement of chemotherapy sensitivity in tumor cells, which was further advanced by blocking CXCR4-PI3K/Akt/mTOR signaling. This study also documented the critical role of silencing CXCR4 in sensitizing ovarian CSCs to chemotherapy. Thus, targeting CXCR4 to suppress EOC progression, specifically in combination with paclitaxel (PTX) treatment, may have clinical application value.
Keywords: CSCs; CXCR4; PI3K/Akt/mTOR; PTX; ovarian cancer.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

