Novel Fluorescent Nanocellulose Hydrogel Based on Nanocellulose and Carbon Dots for Detection and Removal of Heavy Metal Ions in Water
- PMID: 35681368
- PMCID: PMC9180768
- DOI: 10.3390/foods11111619
Novel Fluorescent Nanocellulose Hydrogel Based on Nanocellulose and Carbon Dots for Detection and Removal of Heavy Metal Ions in Water
Abstract
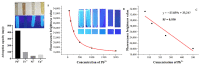
Water is an important raw material in the food production process. Maintaining the quality and safety of water is very important in the food field. In this study, a simple novel fluorescent nanocellulose hydrogel (FNH) was prepared for the detection and removal of heavy metals (Fe3+ and Pb2+) in aqueous solutions based on carbon dots (CDs). The CDs were grafted onto the carboxylated nanocellulose (CNC) by the EDC/NHS coupling method, and then the nanocellulose (NC), CNC, and FNH were characterized by FTIR analysis. The effect of adsorption environment on FNH adsorption capacity was also investigated. After carboxylation and grafting of CDs, the adsorption capacity of nanocellulose to Fe3+ and Pb2+ was greatly improved, and it was also allowed to make fast visual responses to Fe3+ as an optical sensor to determine the concentration of Fe3+ through the visual signal. Static adsorption experiment demonstrated that the removal rate of Fe3+ and Pb2+ by FNH exceeded 69.4% and 98.2%, and the adsorption capacity amount reached 98.3 mg/g and 442.0 mg/g. At the same time, due to the fluorescence quenching effect of Fe3+, FNH could also be used for the detection of Fe3+ concentration in aqueous solution, and the limit of detection (LOD) could reach 62.5 mg/L.
Keywords: adsorption; fluorescent nanocellulose hydrogel; fluorescent sensor; heavy metals ion; nanocellulose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Gao Q., Xu J., Bu X.H. Recent advances about metal-organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2018;378:17–31. doi: 10.1016/j.ccr.2018.03.015. - DOI
-
- Lèbre E., Corder G., Golev A. Sustainable practices in the management of mining waste: A focus on the mineral resource. Miner. Eng. 2017;107:34–42. doi: 10.1016/j.mineng.2016.12.004. - DOI
-
- Baghbadorani N.B., Behzad T., Etesami N., Heidarian P. Removal of Cu2+ ions by cellulose nanofibers-assisted starch-g-poly (acrylic acid) superadsorbent hydrogels. Compos. B Eng. 2019;176:107084. doi: 10.1016/j.compositesb.2019.107084. - DOI
LinkOut - more resources
Full Text Sources

