Leonurine Protects Bone Mesenchymal Stem Cells from Oxidative Stress by Activating Mitophagy through PI3K/Akt/mTOR Pathway
- PMID: 35681421
- PMCID: PMC9179429
- DOI: 10.3390/cells11111724
Leonurine Protects Bone Mesenchymal Stem Cells from Oxidative Stress by Activating Mitophagy through PI3K/Akt/mTOR Pathway
Abstract
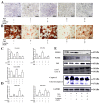
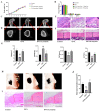
Osteoporosis bears an imbalance between bone formation and resorption, which is strongly related to oxidative stress. The function of leonurine on bone marrow-derived mesenchymal stem cells (BMSCs) under oxidative stress is still unclear. Therefore, this study was aimed at identifying the protective effect of leonurine on H2O2 stimulated rat BMSCs. We found that leonurine can alleviate cell apoptosis and promote the differentiation ability of rat BMSCs induced by oxidative stress at an appropriate concentration at 10 μM. Meanwhile, the intracellular ROS level and the level of the COX2 and NOX4 mRNA decreased after leonurine treatment in vitro. The ATP level and mitochondrial membrane potential were upregulated after leonurine treatment. The protein level of PINK1 and Parkin showed the same trend. The mitophage in rat BMSCs blocked by 3-MA was partially rescued by leonurine. Bioinformatics analysis and leonurine-protein coupling provides a strong direct combination between leonurine and the PI3K protein at the position of Asp841, Glu880, Val882. In conclusion, leonurine protects the proliferation and differentiation of BMSCs from oxidative stress by activating mitophagy, which depends on the PI3K/Akt/mTOR pathway. The results showed that leonurine may have potential usage in osteoporosis and bone defect repair in osteoporosis patients.
Keywords: leonurine; mitophagy; osteoporosis.
Conflict of interest statement
All authors state that they have no conflict of interest.
Figures

Similar articles
-
Ebselen rescues oxidative-stress-suppressed osteogenic differentiation of bone-marrow-derived mesenchymal stem cells via an antioxidant effect and the PI3K/Akt pathway.J Trace Elem Med Biol. 2019 Sep;55:64-70. doi: 10.1016/j.jtemb.2019.06.002. Epub 2019 Jun 10. J Trace Elem Med Biol. 2019. PMID: 31345368
-
Quercetin protects rat BMSCs from oxidative stress via ferroptosis.J Mol Endocrinol. 2022 Aug 24;69(3):401-413. doi: 10.1530/JME-22-0086. Print 2022 Oct 1. J Mol Endocrinol. 2022. PMID: 35900382
-
Leonurine Promotes the Osteoblast Differentiation of Rat BMSCs by Activation of Autophagy via the PI3K/Akt/mTOR Pathway.Front Bioeng Biotechnol. 2021 Feb 23;9:615191. doi: 10.3389/fbioe.2021.615191. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33708763 Free PMC article.
-
Research on the role and mechanism of the PI3K/Akt/mTOR signalling pathway in osteoporosis.Front Endocrinol (Lausanne). 2025 May 12;16:1541714. doi: 10.3389/fendo.2025.1541714. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40421249 Free PMC article. Review.
-
The Complexity of Targeting PI3K-Akt-mTOR Signalling in Human Acute Myeloid Leukaemia: The Importance of Leukemic Cell Heterogeneity, Neighbouring Mesenchymal Stem Cells and Immunocompetent Cells.Molecules. 2016 Nov 11;21(11):1512. doi: 10.3390/molecules21111512. Molecules. 2016. PMID: 27845732 Free PMC article. Review.
Cited by
-
Imbalance of early-life vitamin D intake targets ROS-mediated crosstalk between mitochondrial dysfunction and differentiation potential of MSCs associated the later obesity.Stem Cell Res Ther. 2024 Aug 13;15(1):252. doi: 10.1186/s13287-024-03860-8. Stem Cell Res Ther. 2024. PMID: 39135105 Free PMC article.
-
Comprehensive review of leonurine: harnessing its therapeutic potential for chronic diseases.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr 9. doi: 10.1007/s00210-025-04087-x. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40202674 Review.
-
Exosomes from high-altitude cerebral edema patients induce cognitive dysfunction by altering oxidative stress responses in mice.Transl Psychiatry. 2025 Jul 22;15(1):253. doi: 10.1038/s41398-025-03469-2. Transl Psychiatry. 2025. PMID: 40695777 Free PMC article.
-
Leukemia Inhibitory Factor Facilitates Self-Renewal and Differentiation and Attenuates Oxidative Stress of BMSCs by Activating PI3K/AKT Signaling.Oxid Med Cell Longev. 2022 Sep 5;2022:5772509. doi: 10.1155/2022/5772509. eCollection 2022. Oxid Med Cell Longev. 2022. Retraction in: Oxid Med Cell Longev. 2023 Jun 21;2023:9819142. doi: 10.1155/2023/9819142. PMID: 36105481 Free PMC article. Retracted.
-
Leonurine: a comprehensive review of pharmacokinetics, pharmacodynamics, and toxicology.Front Pharmacol. 2024 Jul 19;15:1428406. doi: 10.3389/fphar.2024.1428406. eCollection 2024. Front Pharmacol. 2024. PMID: 39101131 Free PMC article. Review.
References
-
- Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group. Volume 843. World Health Organization; Geneva, Switzerland: 1994. pp. 1–129. (WHO Technical Report Series). - PubMed
-
- Wright N.C., Looker A.C., Saag K.G., Curtis J.R., Delzell E.S., Randall S., Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 2014;29:2520–2526. doi: 10.1002/jbmr.2269. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

