Alpha-Synuclein Aggregation Pathway in Parkinson's Disease: Current Status and Novel Therapeutic Approaches
- PMID: 35681426
- PMCID: PMC9179656
- DOI: 10.3390/cells11111732
Alpha-Synuclein Aggregation Pathway in Parkinson's Disease: Current Status and Novel Therapeutic Approaches
Abstract
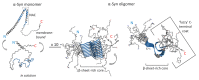
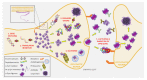
Following Alzheimer's, Parkinson's disease (PD) is the second-most common neurodegenerative disorder, sharing an unclear pathophysiology, a multifactorial profile, and massive social costs worldwide. Despite this, no disease-modifying therapy is available. PD is tightly associated with α-synuclein (α-Syn) deposits, which become organised into insoluble, amyloid fibrils. As a typical intrinsically disordered protein, α-Syn adopts a monomeric, random coil conformation in an aqueous solution, while its interaction with lipid membranes drives the transition of the molecule part into an α-helical structure. The central unstructured region of α-Syn is involved in fibril formation by converting to well-defined, β-sheet rich secondary structures. Presently, most therapeutic strategies against PD are focused on designing small molecules, peptides, and peptidomimetics that can directly target α-Syn and its aggregation pathway. Other approaches include gene silencing, cell transplantation, stimulation of intracellular clearance with autophagy promoters, and degradation pathways based on immunotherapy of amyloid fibrils. In the present review, we sum marise the current advances related to α-Syn aggregation/neurotoxicity. These findings present a valuable arsenal for the further development of efficient, nontoxic, and non-invasive therapeutic protocols for disease-modifying therapy that tackles disease onset and progression in the future.
Keywords: disaggregators; high throughput anti-aggregation drug screening; intrinsically disordered proteins; protein misfolding; rationally designed peptidomimetics; structure/function relationship; synucleinopathies; α-synuclein oligomers and fibrils.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. All authors have read and agreed to the published version of the manuscript.
Figures

References
-
- Kouli A., Torsney K.M., Kuan W.L. Parkinson’s disease: Etiology, neuropathology, and pathogenesis. In: Stoker T.B., Greenland J.C., editors. Parkinson’s Disease: Pathogenesis and Clinical Aspects. Exon Publications; Brisbane, Australia: 2018. pp. 3–26.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

