Membrane Progesterone Receptors (mPRs, PAQRs): Review of Structural and Signaling Characteristics
- PMID: 35681480
- PMCID: PMC9179843
- DOI: 10.3390/cells11111785
Membrane Progesterone Receptors (mPRs, PAQRs): Review of Structural and Signaling Characteristics
Abstract
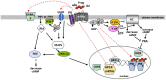
The role of membrane progesterone receptors (mPRs), which belong to the progestin and adipoQ receptor (PAQR) family, in mediating rapid, nongenomic (non-classical) progestogen actions has been extensively studied since their identification 20 years ago. Although the mPRs have been implicated in progestogen regulation of numerous reproductive and non-reproductive functions in vertebrates, several critical aspects of their structure and signaling functions have been unresolved until recently and remain the subject of considerable debate. This paper briefly reviews recent developments in our understanding of the structure and functional characteristics of mPRs. The proposed membrane topology of mPRα, the structure of its ligand-binding site, and the binding affinities of steroids were predicted from homology modeling based on the structures of other PAQRs, adiponectin receptors, and confirmed by mutational analysis and ligand-binding assays. Extensive data demonstrating that mPR-dependent progestogen regulation of intracellular signaling through mPRs is mediated by activation of G proteins are reviewed. Close association of mPRα with progesterone membrane receptor component 1 (PGRMC1), its role as an adaptor protein to mediate cell-surface expression of mPRα and mPRα-dependent progestogen signaling has been demonstrated in several vertebrate models. In addition, evidence is presented that mPRs can regulate the activity of other hormone receptors.
Keywords: 2nd messengers; G-protein; PAQR7; PGRMC1; ligand-binding domain; mPRα; modeling.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions.Front Neuroendocrinol. 2008 May;29(2):292-312. doi: 10.1016/j.yfrne.2008.01.001. Epub 2008 Feb 1. Front Neuroendocrinol. 2008. PMID: 18343488 Free PMC article. Review.
-
Membrane progesterone receptors on the cell membrane: A review highlighting potential export motifs in mPRα regulating its trafficking to the cell surface.Steroids. 2023 Nov;199:109295. doi: 10.1016/j.steroids.2023.109295. Epub 2023 Aug 7. Steroids. 2023. PMID: 37558174 Review.
-
Functions of Membrane Progesterone Receptors (mPRs, PAQRs) in Nonreproductive Tissues.Endocrinology. 2022 Oct 11;163(11):bqac147. doi: 10.1210/endocr/bqac147. Endocrinology. 2022. PMID: 36041040
-
Enhancement of cell surface expression and receptor functions of membrane progestin receptor α (mPRα) by progesterone receptor membrane component 1 (PGRMC1): evidence for a role of PGRMC1 as an adaptor protein for steroid receptors.Endocrinology. 2014 Mar;155(3):1107-19. doi: 10.1210/en.2013-1991. Epub 2014 Jan 1. Endocrinology. 2014. PMID: 24424068 Free PMC article.
-
Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins.Endocrinology. 2007 Feb;148(2):705-18. doi: 10.1210/en.2006-0974. Epub 2006 Nov 2. Endocrinology. 2007. PMID: 17082257
Cited by
-
Allopregnanolone: Metabolism, Mechanisms of Action, and Its Role in Cancer.Int J Mol Sci. 2022 Dec 29;24(1):560. doi: 10.3390/ijms24010560. Int J Mol Sci. 2022. PMID: 36614002 Free PMC article. Review.
-
PGRMC1: An enigmatic heme-binding protein.Pharmacol Ther. 2023 Jan;241:108326. doi: 10.1016/j.pharmthera.2022.108326. Epub 2022 Dec 1. Pharmacol Ther. 2023. PMID: 36463977 Free PMC article. Review.
-
Unraveling the Dynamics of Estrogen and Progesterone Signaling in the Endometrium: An Overview.Cells. 2024 Jul 23;13(15):1236. doi: 10.3390/cells13151236. Cells. 2024. PMID: 39120268 Free PMC article. Review.
-
Sex differences in blood pressure regulation and hypertension: renal, hemodynamic, and hormonal mechanisms.Physiol Rev. 2024 Jan 1;104(1):199-251. doi: 10.1152/physrev.00041.2022. Epub 2023 Jul 21. Physiol Rev. 2024. PMID: 37477622 Free PMC article. Review.
-
Membrane Progesterone Receptors (mPRs/PAQRs) Are Going beyond Its Initial Definitions.Membranes (Basel). 2023 Feb 22;13(3):260. doi: 10.3390/membranes13030260. Membranes (Basel). 2023. PMID: 36984647 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

