Stem Cell-Based Regenerative Therapy and Derived Products in COPD: A Systematic Review and Meta-Analysis
- PMID: 35681492
- PMCID: PMC9180461
- DOI: 10.3390/cells11111797
Stem Cell-Based Regenerative Therapy and Derived Products in COPD: A Systematic Review and Meta-Analysis
Abstract
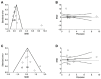
COPD is an incurable disorder, characterized by a progressive alveolar tissue destruction and defective mechanisms of repair and defense leading to emphysema. Currently, treatment for COPD is exclusively symptomatic; therefore, stem cell-based therapies represent a promising therapeutic approach to regenerate damaged structures of the respiratory system and restore lung function. The aim of this study was to provide a quantitative synthesis of the efficacy profile of stem cell-based regenerative therapies and derived products in COPD patients. A systematic review and meta-analysis was performed according to PRISMA-P. Data from 371 COPD patients were extracted from 11 studies. Active treatments elicited a strong tendency towards significance in FEV1 improvement (+71 mL 95% CI -2−145; p = 0.056) and significantly increased 6MWT (52 m 95% CI 18−87; p < 0.05) vs. baseline or control. Active treatments did not reduce the risk of hospitalization due to acute exacerbations (RR 0.77 95% CI 0.40−1.49; p > 0.05). This study suggests that stem cell-based regenerative therapies and derived products may be effective to treat COPD patients, but the current evidence comes from small clinical trials. Large and well-designed randomized controlled trials are needed to really quantify the beneficial impact of stem cell-based regenerative therapy and derived products in COPD.
Keywords: COPD; mesenchymal stem cells; meta-analysis; regenerative.
Conflict of interest statement
L.C. has no conflict of interest to declare; M.A. has no conflict of interest to declare; A.F. has no conflict of interest to declare; F.C. has no conflict of interest to declare; M.C. has no conflict of interest to declare; P.R. has no conflict of interest to declare.; A.C. has no conflict of interest to declare.
Figures



References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for Prevention, Diagnosis and Management of Chronic Obstructive Pulmonary Disease; 2022 Report. [(accessed on 5 April 2022)]. Available online: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22....
-
- World Health Organization WHO—The Top 10 Causes of Death. [(accessed on 5 April 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

