Analysis of Leukocyte Recruitment in Continuous Veno-Venous Hemofiltration with Regional Citrate vs. Systemic Heparin Anticoagulation
- PMID: 35681510
- PMCID: PMC9180305
- DOI: 10.3390/cells11111815
Analysis of Leukocyte Recruitment in Continuous Veno-Venous Hemofiltration with Regional Citrate vs. Systemic Heparin Anticoagulation
Abstract
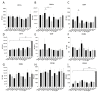
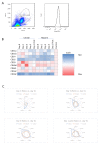
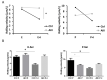
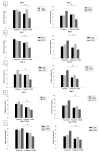
Acute kidney injury (AKI) is a frequent complication in critically ill patients. Supportive treatment of AKI patients is based on renal-replacement therapy, including continuous veno-venous hemofiltration (CVVH). To limit clotting events on extracorporeal surfaces, anticoagulants are administered, including systemic heparin and local citrate. The differential and comparative effects of these anticoagulants on leukocyte function in acute kidney injury patients are, so far, insufficiently understood. In this bio-add-on-study, AKI patients were randomized as part of a parallel-group trial to either systemic heparin or regional citrate anticoagulation. Patient samples were collected upon inclusion, prior to CVVH initiation at day 0, day 1, day 3 and day 5, following CVVH initiation, and one day after cessation of CVVH, then immediately analyzed. Flow cytometric assessment of surface-receptor molecules was conducted. Whole-blood-perfused human microfluidic chambers were used for the analysis of neutrophil rolling and adhesion. Acute kidney injury was associated with significant changes in the surface expression of CD182 and CD16 throughout CVVH treatment, independent of the anticoagulation regime. AKI furthermore abrogated selectin-induced slow leukocyte rolling and diminished chemokine-induced leukocyte arrest. Subgroup analyses of citrate vs. heparin treatment showed no significant differences between groups, independent of the duration of CVVH treatment. CD182 and CD16 expression remained low in both groups throughout CVVH therapy. These data confirm that AKI impairs selectin-mediated leukocyte slow rolling and chemokine-induced leukocyte arrest in vitro. Systemic heparin or local citrate anticoagulation have no differential effect on the leukocyte recruitment steps examined in this study.
Keywords: CVVH; acute kidney injury; anticoagulation; citrate; heparin; inflammation; leukocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
[Effects of regional citrate anticoagulation in continuous veno-venous hemofiltration of severe burn patients].Zhonghua Shao Shang Za Zhi. 2021 Dec 20;37(12):1137-1142. doi: 10.3760/cma.j.cn501120-20200816-00381. Zhonghua Shao Shang Za Zhi. 2021. PMID: 34839601 Free PMC article. Clinical Trial. Chinese.
-
Citrate anticoagulation versus systemic heparinisation in continuous venovenous hemofiltration in critically ill patients with acute kidney injury: a multi-center randomized clinical trial.Crit Care. 2014 Aug 16;18(4):472. doi: 10.1186/s13054-014-0472-6. Crit Care. 2014. PMID: 25128022 Free PMC article. Clinical Trial.
-
Regional citrate versus systemic heparin for anticoagulation in critically ill patients on continuous venovenous haemofiltration: a prospective randomized multicentre trial.Nephrol Dial Transplant. 2011 Jan;26(1):232-9. doi: 10.1093/ndt/gfq575. Epub 2010 Sep 27. Nephrol Dial Transplant. 2011. PMID: 20876598 Clinical Trial.
-
Calcium-Citrate Anticoagulation during Continuous Renal Replacement Therapy in Patients with Metformin Intoxication: A Case Series, Mathematical Estimation of Citrate Accumulation, and Literature Review.Blood Purif. 2023;52(9-10):802-811. doi: 10.1159/000531953. Epub 2023 Sep 6. Blood Purif. 2023. PMID: 37673054 Review.
-
Heparin versus citrate for anticoagulation in critically ill patients treated with continuous renal replacement therapy.Nurs Crit Care. 2009 Jul-Aug;14(4):191-9. doi: 10.1111/j.1478-5153.2009.00339.x. Nurs Crit Care. 2009. PMID: 19531037 Review.
Cited by
-
Anticoagulation options for continuous renal replacement therapy in critically ill patients: a systematic review and network meta-analysis of randomized controlled trials.Crit Care. 2023 Jun 7;27(1):222. doi: 10.1186/s13054-023-04519-1. Crit Care. 2023. PMID: 37287084 Free PMC article.
References
-
- Zarbock A., Kellum J.A., Schmidt C., Van Aken H., Wempe C., Pavenstadt H., Boanta A., Gerss J., Meersch M. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients with Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA. 2016;315:2190–2199. doi: 10.1001/jama.2016.5828. - DOI - PubMed
-
- STARRT-AKI Investigators. Canadian Critical Care Trials Group. Australian and New Zealand Intensive Care Society Clinical Trials Group. United Kingdom Critical Care Research Group. Canadian Nephrology Trials Network. Irish Critical Care Trials Group. Bagshaw S.M., Wald R., Adhikari N.K.J., Bellomo R., et al. Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N. Engl. J. Med. 2020;383:240–251. doi: 10.1056/NEJMoa2000741. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

