Hematopoietic Stem Cell Gene-Addition/Editing Therapy in Sickle Cell Disease
- PMID: 35681538
- PMCID: PMC9180595
- DOI: 10.3390/cells11111843
Hematopoietic Stem Cell Gene-Addition/Editing Therapy in Sickle Cell Disease
Abstract
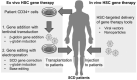
Autologous hematopoietic stem cell (HSC)-targeted gene therapy provides a one-time cure for various genetic diseases including sickle cell disease (SCD) and β-thalassemia. SCD is caused by a point mutation (20A > T) in the β-globin gene. Since SCD is the most common single-gene disorder, curing SCD is a primary goal in HSC gene therapy. β-thalassemia results from either the absence or the reduction of β-globin expression, and it can be cured using similar strategies. In HSC gene-addition therapy, patient CD34+ HSCs are genetically modified by adding a therapeutic β-globin gene with lentiviral transduction, followed by autologous transplantation. Alternatively, novel gene-editing therapies allow for the correction of the mutated β-globin gene, instead of addition. Furthermore, these diseases can be cured by γ-globin induction based on gene addition/editing in HSCs. In this review, we discuss HSC-targeted gene therapy in SCD with gene addition as well as gene editing.
Keywords: gene editing; gene therapy; hematopoietic stem cells; in vivo gene therapy; sickle cell disease; transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical