Repurposing Vitamin C for Cancer Treatment: Focus on Targeting the Tumor Microenvironment
- PMID: 35681589
- PMCID: PMC9179307
- DOI: 10.3390/cancers14112608
Repurposing Vitamin C for Cancer Treatment: Focus on Targeting the Tumor Microenvironment
Abstract
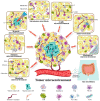
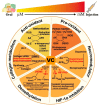
Based on the enhanced knowledge on the tumor microenvironment (TME), a more comprehensive treatment landscape for targeting the TME has emerged. This microenvironment provides multiple therapeutic targets due to its diverse characteristics, leading to numerous TME-targeted strategies. With multifaced activities targeting tumors and the TME, vitamin C is renown as a promising candidate for combination therapy. In this review, we present new advances in how vitamin C reshapes the TME in the immune, hypoxic, metabolic, acidic, neurological, mechanical, and microbial dimensions. These findings will open new possibilities for multiple therapeutic avenues in the fight against cancer. We also review the available preclinical and clinical evidence of vitamin C combined with established therapies, highlighting vitamin C as an adjuvant that can be exploited for novel therapeutics. Finally, we discuss unresolved questions and directions that merit further investigation.
Keywords: anti-immunity; dietary intervention; drug repurposing; tumor microenvironment; vitamin C.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures

References
-
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

