Unveiling the Molecular Mechanisms Driving the Capsaicin-Induced Immunomodulatory Effects on PD-L1 Expression in Bladder and Renal Cancer Cell Lines
- PMID: 35681623
- PMCID: PMC9179445
- DOI: 10.3390/cancers14112644
Unveiling the Molecular Mechanisms Driving the Capsaicin-Induced Immunomodulatory Effects on PD-L1 Expression in Bladder and Renal Cancer Cell Lines
Abstract
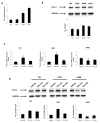
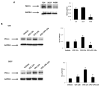
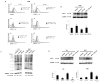
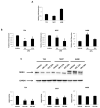
The blockade of the PD-L1/PD-1 immune checkpoint has promising efficacy in cancer treatment. However, few patients with bladder cancer (BC) or renal cell carcinoma (RCC) respond to this approach. Thus, it is important to implement a strategy to stimulate the immune anti-tumor response. In this scenario, our study evaluated the effects of a low capsaicin (CPS) dose in BC and RCC cell lines. Western blot, qRT-PCR and confocal microscopy were used to assess PD-L1 mRNA and protein expression. Alterations to the cellular oxidative status and changes to the antioxidant NME4 levels, mRNA modulation of cytokines, growth factors, transcriptional factors and oncogene, and the activation of Stat1/Stat3 pathways were examined using Western blot, cytofluorimetry and qRT-PCR profiling assays. In BC, CPS triggers an altered stress oxidative-mediated DNA double-strand break response and increases the PD-L1 expression. On the contrary, in RCC, CPS, by stimulating an efficient DNA damage repair response, thus triggering protein carbonylation, reduces the PD-L1 expression. Overall, our results show that CPS mediates a multi-faceted approach. In modulating PD-L1 expression, there is a rationale for CPS exploitation as a stimulus that increases BC cells' response to immunotherapy or as an immune adjuvant to improve the efficacy of the conventional therapy in RCC patients.
Keywords: PD-L1; bladder cancer; capsaicin; genitourinary cancer; immunotherapy; renal cell carcinoma.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
PD-L1 expression in bladder cancer: Which scoring algorithm in what tissue?Urol Oncol. 2021 Oct;39(10):734.e1-734.e10. doi: 10.1016/j.urolonc.2021.06.001. Epub 2021 Jul 11. Urol Oncol. 2021. PMID: 34261585
-
Cisplatin contributes to programmed death-ligand 1 expression in bladder cancer through ERK1/2-AP-1 signaling pathway.Biosci Rep. 2019 Sep 6;39(9):BSR20190362. doi: 10.1042/BSR20190362. Print 2019 Sep 30. Biosci Rep. 2019. PMID: 31341011 Free PMC article.
-
ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation.Theranostics. 2021 Jan 19;11(7):3392-3416. doi: 10.7150/thno.52435. eCollection 2021. Theranostics. 2021. PMID: 33537094 Free PMC article.
-
PD-1 blockade therapy in renal cell carcinoma: current studies and future promises.Cancer Treat Rev. 2015 Feb;41(2):114-21. doi: 10.1016/j.ctrv.2014.12.013. Epub 2015 Jan 6. Cancer Treat Rev. 2015. PMID: 25586601 Review.
-
Dual inhibition of STAT1 and STAT3 activation downregulates expression of PD-L1 in human breast cancer cells.Expert Opin Ther Targets. 2018 Jun;22(6):547-557. doi: 10.1080/14728222.2018.1471137. Epub 2018 May 2. Expert Opin Ther Targets. 2018. PMID: 29702007 Review.
Cited by
-
Immunomodulatory Prodrug Micelles Imitate Mild Heat Effects to Reshape Tumor Microenvironment for Enhanced Cancer Immunotherapy.ACS Nano. 2024 Feb 20;18(7):5632-5646. doi: 10.1021/acsnano.3c11186. Epub 2024 Feb 12. ACS Nano. 2024. PMID: 38344992 Free PMC article.
-
Preparation of capsaicin-loaded ultrafine fiber film and its application in the treatment of oral ulcers in rats.Sci Rep. 2023 Aug 25;13(1):13941. doi: 10.1038/s41598-023-40375-3. Sci Rep. 2023. PMID: 37626141 Free PMC article.
-
Is the Triggering of PD-L1 Dimerization a Potential Mechanism for Food-Derived Small Molecules in Cancer Immunotherapy? A Study by Molecular Dynamics.Int J Mol Sci. 2023 Jan 11;24(2):1413. doi: 10.3390/ijms24021413. Int J Mol Sci. 2023. PMID: 36674929 Free PMC article.
-
Spice Up Your Kidney: A Review on the Effects of Capsaicin in Renal Physiology and Disease.Int J Mol Sci. 2024 Jan 8;25(2):791. doi: 10.3390/ijms25020791. Int J Mol Sci. 2024. PMID: 38255865 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

