Crosstalk of Epigenetic and Metabolic Signaling Underpinning Glioblastoma Pathogenesis
- PMID: 35681635
- PMCID: PMC9179868
- DOI: 10.3390/cancers14112655
Crosstalk of Epigenetic and Metabolic Signaling Underpinning Glioblastoma Pathogenesis
Abstract
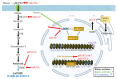
Metabolic alterations in neoplastic cells have recently gained increasing attention as a main topic of research, playing a crucial regulatory role in the development and progression of tumors. The interplay between epigenetic modifications and metabolic pathways in glioblastoma cells has emerged as a key pathogenic area with great potential for targeted therapy. Epigenetic mechanisms have been demonstrated to affect main metabolic pathways, such as glycolysis, pentose phosphate pathway, gluconeogenesis, oxidative phosphorylation, TCA cycle, lipid, and glutamine metabolism by modifying key regulatory genes. Although epigenetic modifications can primarily promote the activity of metabolic pathways, they may also exert an inhibitory role. In this way, they participate in a complex network of interactions that regulate the metabolic behavior of malignant cells, increasing their heterogeneity and plasticity. Herein, we discuss the main epigenetic mechanisms that regulate the metabolic pathways in glioblastoma cells and highlight their targeting potential against tumor progression.
Keywords: DNA; Krebs cycle; TCA cycle; acetylation; glioblastoma; glioma; gluconeogenesis; glutamine; glycolysis; histones; methylation; microRNAs; oxidative phosphorylation; pentose phosphate pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Targeting post-translational histone modifying enzymes in glioblastoma.Pharmacol Ther. 2021 Apr;220:107721. doi: 10.1016/j.pharmthera.2020.107721. Epub 2020 Nov 2. Pharmacol Ther. 2021. PMID: 33144118 Review.
-
Interplay Among Metabolism, Epigenetic Modifications, and Gene Expression in Cancer.Front Cell Dev Biol. 2021 Dec 24;9:793428. doi: 10.3389/fcell.2021.793428. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 35004688 Free PMC article. Review.
-
Metabolic dysregulation of tricarboxylic acid cycle and oxidative phosphorylation in glioblastoma.Rev Neurosci. 2024 Jun 7;35(7):813-838. doi: 10.1515/revneuro-2024-0054. Print 2024 Oct 28. Rev Neurosci. 2024. PMID: 38841811 Review.
-
Crosstalk between macrophages and immunometabolism and their potential roles in tissue repair and regeneration.Heliyon. 2024 Sep 18;10(18):e38018. doi: 10.1016/j.heliyon.2024.e38018. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39381218 Free PMC article. Review.
-
DNA methylation, histone acetylation and methylation of epigenetic modifications as a therapeutic approach for cancers.Cancer Lett. 2016 Apr 10;373(2):185-92. doi: 10.1016/j.canlet.2016.01.036. Epub 2016 Jan 22. Cancer Lett. 2016. PMID: 26808576 Review.
Cited by
-
From Pathogens to Cancer: Are Cancer Cells Evolved Mitochondrial Super Cells?Diagnostics (Basel). 2023 Feb 20;13(4):813. doi: 10.3390/diagnostics13040813. Diagnostics (Basel). 2023. PMID: 36832301 Free PMC article.
-
Impact of Solute Carrier Transporters in Glioma Pathology: A Comprehensive Review.Int J Mol Sci. 2023 May 28;24(11):9393. doi: 10.3390/ijms24119393. Int J Mol Sci. 2023. PMID: 37298344 Free PMC article. Review.
-
Emerging Role of the Slit/Roundabout (Robo) Signaling Pathway in Glioma Pathogenesis and Potential Therapeutic Options.Biomolecules. 2024 Sep 29;14(10):1231. doi: 10.3390/biom14101231. Biomolecules. 2024. PMID: 39456164 Free PMC article. Review.
-
Epigenetics and Metabolism Reprogramming Interplay into Glioblastoma: Novel Insights on Immunosuppressive Mechanisms.Antioxidants (Basel). 2023 Jan 18;12(2):220. doi: 10.3390/antiox12020220. Antioxidants (Basel). 2023. PMID: 36829778 Free PMC article. Review.
-
Impact of Histone Lysine Methyltransferase SUV4-20H2 on Cancer Onset and Progression with Therapeutic Potential.Int J Mol Sci. 2024 Feb 21;25(5):2498. doi: 10.3390/ijms25052498. Int J Mol Sci. 2024. PMID: 38473745 Free PMC article. Review.
References
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
-
- Kanderi T., Gupta V. Glioblastoma Multiforme. StatPearls Publishing; Treasure Island, UK: 2021. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

