Ficus dubia Latex Extract Induces Cell Cycle Arrest and Apoptosis by Regulating the NF-κB Pathway in Inflammatory Human Colorectal Cancer Cell Lines
- PMID: 35681644
- PMCID: PMC9179257
- DOI: 10.3390/cancers14112665
Ficus dubia Latex Extract Induces Cell Cycle Arrest and Apoptosis by Regulating the NF-κB Pathway in Inflammatory Human Colorectal Cancer Cell Lines
Abstract
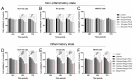
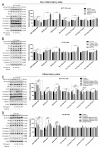
Colorectal cancer is one of the most diagnosed cancers that is associated with inflammation. Ficus dubia latex is recognized as a remedy with various therapeutic effects in traditional medicine, including anti-inflammatory and antioxidant activity. The present study aims to compare the anti-tumor activity of Ficus dubia latex extract (FDLE) against HCT-116 and HT-29 human colorectal cancer cell lines in normal and inflammatory condition and explore its mechanism of action. FDLE exhibited remarkable antiproliferative activity against HCT-116 and HT-29 colorectal cancer cell lines in both conditions using MTT and colony formation assays and more effective anti-proliferation was observed in inflammatory condition. Mechanistically, FDLE induced cell cycle arrest at G0/G1 phase by down-regulating NF-κB, cyclin D1, CDK4 and up-regulatingp21 in both cell in normal condition. In inflammatory condition, FDLE not only exhibited stronger induction of cell cycle arrest in both cells by down-regulating NF-κB, cyclin D1, CDK4 and down-regulating p21, but also selectively induced apoptosis in HCT-116 cells by down-regulating NF-κB and Bcl-xl and up-regulating Bid, Bak, cleaved caspase-7 and caspase-3 through stronger ability to regulate these proteins. Our results demonstrated that the phytochemical agent in the latex of Ficus dubia could potential be used for treatment and prevention of human colorectal cancer, especially in inflammation-induced hyperproliferation progression.
Keywords: Ficus dubia latex extract (FDLE); NF-κB pathway; apoptosis; cell cycle arrest; colorectal cancer; inflammation; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ieda T., Tazawa H., Okabayashi H., Yano S., Shigeyasu K., Kuroda S., Ohara T., Noma K., Kishimoto H., Nishizaki M. Visualization of epithelial-mesenchymal transition in an inflammatory microenvironment–colorectal cancer network. Sci. Rep. 2019;9:16378. doi: 10.1038/s41598-019-52816-z. - DOI - PMC - PubMed
-
- Yoon S.-W. Systemic inflammatory response as a prognostic factor in patients with cancer. J. Korean Tradit. Oncol. 2012;17:1–7.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

