PET-CT in Clinical Adult Oncology: III. Gastrointestinal Malignancies
- PMID: 35681647
- PMCID: PMC9179927
- DOI: 10.3390/cancers14112668
PET-CT in Clinical Adult Oncology: III. Gastrointestinal Malignancies
Abstract
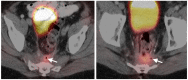
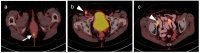
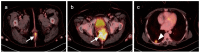
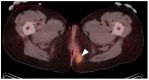
PET-CT is an advanced imaging modality with many oncologic applications, including staging, assessment of response to therapy, restaging and longitudinal surveillance for recurrence. The goal of this series of six review articles is to provide practical information to providers and imaging professionals regarding the best use of PET-CT for specific oncologic indications, and the potential pitfalls and nuances that characterize these applications. In the third of these review articles, key tumor-specific clinical information and representative PET-CT images are provided to outline the role that PET-CT plays in the management of patients with gastrointestinal malignancies. The focus is on the use of 18F fluorodeoxyglucose (FDG), rather than on research radiopharmaceuticals under development. Many different types of gastrointestinal tumors exist, both pediatric and adult. A discussion of the role of FDG PET-CT for all of these is beyond the scope of this review. Rather, this article focuses on the most common adult gastrointestinal malignancies that may be encountered in clinical practice. The information provided here will provide information outlining the appropriate role of PET-CT in the clinical management of patients with gastrointestinal malignancies for healthcare professionals caring for adult cancer patients. It also addresses the nuances and provides interpretive guidance related to PET-CT for imaging providers, including radiologists, nuclear medicine physicians and their trainees.
Keywords: FDG; PET; adrenal carcinoma; anal carcinoma; colorectal cancer pancreatic cancer; esophageal cancer; gastric cancer; hepatobiliary carcinoma; small-bowel carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

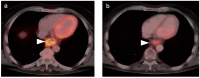
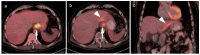
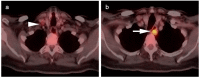
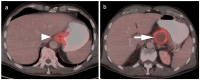
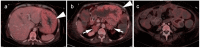
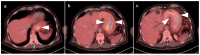
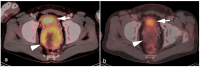
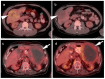
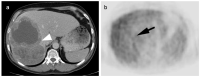
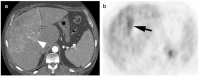
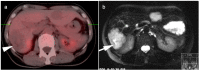
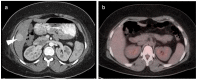
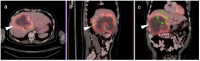
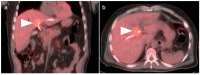
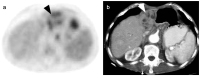
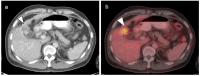
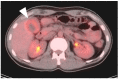
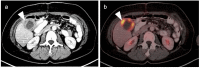
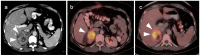
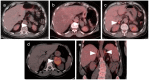
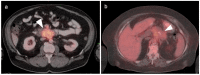
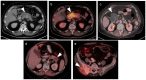
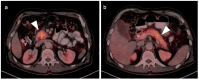
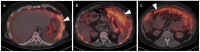
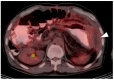
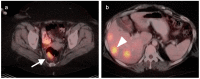
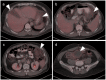
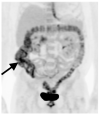
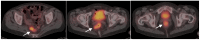
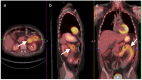
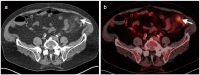
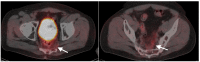
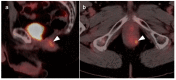
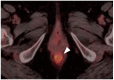
References
-
- Recio-Boiles A., Babiker H.M. Esophageal Cancer. [Updated 21 July 2021]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. [(accessed on 21 July 2021)];2022 January; Available online: https://www.ncbi.nlm.nih.gov/books/NBK459267/
Publication types
LinkOut - more resources
Full Text Sources

