In Silico Investigations of Multi-Drug Adaptive Therapy Protocols
- PMID: 35681680
- PMCID: PMC9179496
- DOI: 10.3390/cancers14112699
In Silico Investigations of Multi-Drug Adaptive Therapy Protocols
Abstract
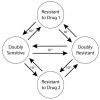
The standard of care for cancer patients aims to eradicate the tumor by killing the maximum number of cancer cells using the maximum tolerated dose (MTD) of a drug. MTD causes significant toxicity and selects for resistant cells, eventually making the tumor refractory to treatment. Adaptive therapy aims to maximize time to progression (TTP), by maintaining sensitive cells to compete with resistant cells. We explored both dose modulation (DM) protocols and fixed dose (FD) interspersed with drug holiday protocols. In contrast to previous single drug protocols, we explored the determinants of success of two-drug adaptive therapy protocols, using an agent-based model. In almost all cases, DM protocols (but not FD protocols) increased TTP relative to MTD. DM protocols worked well when there was more competition, with a higher cost of resistance, greater cell turnover, and when crowded proliferating cells could replace their neighbors. The amount that the drug dose was changed, mattered less. The more sensitive the protocol was to tumor burden changes, the better. In general, protocols that used as little drug as possible, worked best. Preclinical experiments should test these predictions, especially dose modulation protocols, with the goal of generating successful clinical trials for greater cancer control.
Keywords: adaptive therapy; agent-based model; cancer; dose modulation; drug resistance; evolution.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





Similar articles
-
Testing Adaptive Therapy Protocols Using Gemcitabine and Capecitabine in a Preclinical Model of Endocrine-Resistant Breast Cancer.Cancers (Basel). 2024 Jan 6;16(2):257. doi: 10.3390/cancers16020257. Cancers (Basel). 2024. PMID: 38254748 Free PMC article.
-
Testing Adaptive Therapy Protocols using Gemcitabine and Capecitabine on a Mouse Model of Endocrine-Resistant Breast Cancer.bioRxiv [Preprint]. 2023 Sep 22:2023.09.18.558136. doi: 10.1101/2023.09.18.558136. bioRxiv. 2023. Update in: Cancers (Basel). 2024 Jan 06;16(2):257. doi: 10.3390/cancers16020257. PMID: 37781632 Free PMC article. Updated. Preprint.
-
The Challenge of Developing New Therapies for Childhood Cancers.Oncologist. 1997;2(1):I-II. Oncologist. 1997. PMID: 10388032
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
-
The potential clinical promise of 'multimodality' metronomic chemotherapy revealed by preclinical studies of metastatic disease.Cancer Lett. 2017 Aug 1;400:293-304. doi: 10.1016/j.canlet.2017.02.005. Epub 2017 Feb 12. Cancer Lett. 2017. PMID: 28202353 Review.
Cited by
-
Resistance Management for Cancer: Lessons from Farmers.Cancer Res. 2024 Nov 15;84(22):3715-3727. doi: 10.1158/0008-5472.CAN-23-3374. Cancer Res. 2024. PMID: 39356625 Free PMC article. Review.
-
A survey of open questions in adaptive therapy: Bridging mathematics and clinical translation.Elife. 2023 Mar 23;12:e84263. doi: 10.7554/eLife.84263. Elife. 2023. PMID: 36952376 Free PMC article.
-
Testing Adaptive Therapy Protocols Using Gemcitabine and Capecitabine in a Preclinical Model of Endocrine-Resistant Breast Cancer.Cancers (Basel). 2024 Jan 6;16(2):257. doi: 10.3390/cancers16020257. Cancers (Basel). 2024. PMID: 38254748 Free PMC article.
-
Leveraging Cancer Phenotypic Plasticity for Novel Treatment Strategies.J Clin Med. 2024 Jun 5;13(11):3337. doi: 10.3390/jcm13113337. J Clin Med. 2024. PMID: 38893049 Free PMC article.
-
Testing Adaptive Therapy Protocols using Gemcitabine and Capecitabine on a Mouse Model of Endocrine-Resistant Breast Cancer.bioRxiv [Preprint]. 2023 Sep 22:2023.09.18.558136. doi: 10.1101/2023.09.18.558136. bioRxiv. 2023. Update in: Cancers (Basel). 2024 Jan 06;16(2):257. doi: 10.3390/cancers16020257. PMID: 37781632 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources

