Early Development of Ubiquitous Acanthocytosis and Extravascular Hemolysis in Lung Cancer Patients Receiving Alectinib
- PMID: 35681698
- PMCID: PMC9179520
- DOI: 10.3390/cancers14112720
Early Development of Ubiquitous Acanthocytosis and Extravascular Hemolysis in Lung Cancer Patients Receiving Alectinib
Abstract
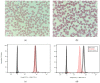
Alectinib is a standard initial treatment for patients with advanced anaplastic lymphoma kinase (ALK) rearranged non-small-cell lung cancer (NSCLC). The current study analyzed a prospective cohort of 24 consecutive alectinib-treated patients and controls in order to comprehensively characterize longitudinal erythrocyte changes under treatment with ALK inhibitors. Upon starting alectinib, all examined patients developed reticulocytosis and abnormal erythrocyte morphology with anisocytosis and a predominance of acanthocytes (64% of red blood cells on average, range 36−100%) in the peripheral blood smear within approximately 2 weeks. Changes were accompanied by a gradual reduction in Eosin-5-maleimide (EMA) binding, which became pathologic (<80% of cells) within 1−2 months in all cases, mimicking an abortive form of hereditary spherocytosis. The latter could be ruled out in 3/3 of analyzed cases by normal sequencing results for the ANK1, EPB42, SLC4A1, SPTA1, or SBTB genes. The direct Coombs test was also negative in 11/11 tested cases. Besides, anemia, increased LDH, and increased bilirubin were noted in a fraction of patients only, ranging between 42 and 68%. Furthermore, haptoglobin decreases were infrequent, occurring in approximately 1/3 of cases only, and mild, with an average value of 0.93 g/L within the normal range of 0.3−2 g/dL, suggesting that hemolysis occurred predominantly in the extravascular compartment, likely due to splenic trapping of the deformed erythrocytes. These changes showed no association with progression-free survival under alectinib or molecular features, i.e., ALK fusion variant or TP53 status of the disease, and resolved upon a switch to an alternative ALK inhibitor. Thus, alectinib induces mild, reversible erythrocyte changes in practically all treated patients, whose most sensitive signs are aberrant red cell morphology in the peripheral smear, a pathologic EMA test, and reactive reticulocytosis. Frank hemolytic anemia is rare, but mild subclinical hemolysis is very frequent and poses differential-diagnostic problems. Alectinib can be continued under the regular control of hemolysis parameters, but the risk of long-term complications, such as cholelithiasis due to increased serum bilirubin in most patients, remains unclear at present.
Keywords: ALK+ NSCLC; acanthocytosis; alectinib; anemia; hemolysis.
Conflict of interest statement
A.S.: advisory board honoraria from BMS, Astra Zeneca, Thermo Fisher, and Novartis; speaker’s honoraria from BMS, Illumina, Astra Zeneca, Novartis, Thermo Fisher, MSD, and Roche; and research funding from Chugai and BMS. M.T.: advisory board honoraria from Novartis, Eli Lilly, BMS, MSD, Roche, Celgene, Takeda, AbbVie, Boehringer Ingelheim, and Pfizer; speaker’s honoraria from Eli Lilly, MSD, Takeda, and Pfizer; research funding from Astra Zeneca, BMS, Celgene, Novartis, Roche, and Takeda; and travel grants from BMS, MSD, Novartis, and Boehringer. P.C.: research funding from Amgen, Astra Zeneca, Boehringer Ingelheim, Merck, Novartis, Roche, and Takeda; and advisory board/lecture fees from Astra Zeneca, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Gilead, Novartis, Pfizer, Roche, and Takeda. All other authors have no conflict of interest to declare.
Figures



Similar articles
-
Changes in Red Cell Morphology and Haematological Laboratory Parameters Associated With Alectinib.J Clin Lab Anal. 2024 Jul;38(13-14):e25089. doi: 10.1002/jcla.25089. J Clin Lab Anal. 2024. PMID: 39129486 Free PMC article.
-
Alectinib-Induced Severe Hemolytic Anemia in a Patient with ALK-Positive Non-Small Cell Lung Cancer: A Case Report.Onco Targets Ther. 2023 Jan 24;16:65-69. doi: 10.2147/OTT.S398375. eCollection 2023. Onco Targets Ther. 2023. PMID: 36718244 Free PMC article.
-
Alectinib induces marked red cell spheroacanthocytosis in a near-ubiquitous fashion and is associated with reduced eosin-5-maleimide binding.Pathology. 2021 Aug;53(5):608-612. doi: 10.1016/j.pathol.2020.10.023. Epub 2021 Feb 19. Pathology. 2021. PMID: 33618863
-
Mixed responses to first-line alectinib in non-small cell lung cancer patients with rare ALK gene fusions: A case series and literature review.J Cell Mol Med. 2021 Oct;25(19):9476-9481. doi: 10.1111/jcmm.16897. Epub 2021 Sep 19. J Cell Mol Med. 2021. PMID: 34541785 Free PMC article. Review.
-
Effect of alectinib versus crizotinib on progression-free survival, central nervous system efficacy and adverse events in ALK-positive non-small cell lung cancer: a systematic review and meta-analysis.Ann Palliat Med. 2020 Jul;9(4):1782-1796. doi: 10.21037/apm-19-643. Epub 2020 Jun 8. Ann Palliat Med. 2020. PMID: 32527124
Cited by
-
Changes in Red Cell Morphology and Haematological Laboratory Parameters Associated With Alectinib.J Clin Lab Anal. 2024 Jul;38(13-14):e25089. doi: 10.1002/jcla.25089. J Clin Lab Anal. 2024. PMID: 39129486 Free PMC article.
-
Lorlatinib and compound mutations in ALK+ large-cell neuroendocrine lung carcinoma: a case report.Cold Spring Harb Mol Case Stud. 2022 Oct 28;8(6):a006234. doi: 10.1101/mcs.a006234. Print 2022 Oct. Cold Spring Harb Mol Case Stud. 2022. PMID: 36207130 Free PMC article.
-
Alectinib-Induced Severe Hemolytic Anemia in a Patient with ALK-Positive Non-Small Cell Lung Cancer: A Case Report.Onco Targets Ther. 2023 Jan 24;16:65-69. doi: 10.2147/OTT.S398375. eCollection 2023. Onco Targets Ther. 2023. PMID: 36718244 Free PMC article.
-
Alectinib-induced Hemolytic Anemia with Positive Direct Antiglobulin Test in a Patient with Lung Adenocarcinoma: A Possible Drug-drug Interaction Effect.Intern Med. 2024 Mar 1;63(5):711-715. doi: 10.2169/internalmedicine.1286-22. Epub 2023 Jul 12. Intern Med. 2024. PMID: 37438141 Free PMC article.
-
Epithelioid inflammatory myofibroblastic sarcoma treated with Alectinib: a case report and literature review.Front Oncol. 2024 Aug 30;14:1412225. doi: 10.3389/fonc.2024.1412225. eCollection 2024. Front Oncol. 2024. PMID: 39281378 Free PMC article.
References
-
- Hanna N.H., Robinson A.G., Temin S., Baker S., Brahmer J.R., Ellis P.M., Gaspar L.E., Haddad R.Y., Hesketh P.J., Jain D., et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2021;39:1040–1091. doi: 10.1200/JCO.20.03570. - DOI - PubMed
-
- Planchard D., Popat S., Kerr K., Novello S., Smit E.F., Faivre-Finn C., Mok T.S., Reck M., van Schil P.E., Hellmann M.D., et al. Metastatic Non-small Cell Lung Cancer: Esmo Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. - DOI - PubMed
-
- Planchard D., Popat S., Kerr K., Novello S., Smit E.F., Faivre-Finn C., Mok T.S., Reck M., van Schil P.E., Hellmann M.D., et al. ESMO Clinical Practice Living Guidelines—Metastatic Non-Small-Cell Lung Cancer. [(accessed on 18 April 2021)]. Available online: https://www.esmo.org/guidelines/lung-and-chest-tumours/clinical-practice....
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

