Treatment Approaches and Outcome of Patients with Neuroendocrine Neoplasia Grade 3 in German Real-World Clinical Practice
- PMID: 35681701
- PMCID: PMC9179270
- DOI: 10.3390/cancers14112718
Treatment Approaches and Outcome of Patients with Neuroendocrine Neoplasia Grade 3 in German Real-World Clinical Practice
Abstract
Background: Neuroendocrine neoplasia grade 3 (NEN G3) represents a rare and heterogeneous cancer type with a poor prognosis. The aim of our study was to analyze real-world data from the German NET Registry with a focus on therapeutic and prognostic aspects.
Methods: NEN G3 patients were identified within the German NET Registry. Demographic data and data on treatments and outcomes were retrieved. Univariate analyses were performed using the Kaplan-Meier-method. Multivariate analysis was performed using a Cox proportional hazard model.
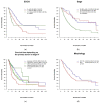
Results: Of 445 included patients, 318 (71.5%) were diagnosed at stage IV. Well-differentiated morphology (NET G3) was described in 31.7%, 60% of cases were classified as neuroendocrine carcinoma (NEC), and the median Ki67 value was 50%. First-line treatment comprised chemotherapy in 43.8%, with differences in the choice of regimen with regard to NET or NEC, and surgery in 41.6% of patients. Median overall survival for the entire cohort was 31 months. Stage, performance status and Ki67 were significant prognostic factors in multivariate analysis.
Conclusions: The survival data of our national registry compare favorably to population-based data, probably mainly because of a relatively low median Ki67 of 50%. Nevertheless, the best first- and second-line approaches for specific subgroups remain unclear, and an international effort to fill these gaps is needed.
Keywords: Ki67; chemotherapy; neuroendocrine carcinoma; neuroendocrine neoplasia; neuroendocrine tumor G3; prognosis.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- Garcia-Carbonero R., Sorbye H., Baudin E., Raymond E., Wiedenmann B., Niederle B., Sedlackova E., Toumpanakis C., Anlauf M., Cwikla J.B., et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology. 2016;103:186–194. doi: 10.1159/000443172. - DOI - PubMed
-
- Lombard-Bohas C., Mitry E., O’Toole D., Louvet C., Pillon D., Cadiot G., Borson-Chazot F., Aparicio T., Ducreux M., Lecomte T., et al. Rougier, and FFCD-ANGH-GERCOR, Thirteen-month registration of patients with gastroenteropancreatic endocrine tumours in France. Neuroendocrinology. 2009;89:217–222. doi: 10.1159/000151562. - DOI - PubMed
-
- Garcia-Carbonero R., Capdevila J., Crespo-Herrero G., Díaz-Pérez J.A., del Prado M.P.M., Orduña V.A., Sevilla-García I., Villabona-Artero C., Beguiristain-Gómez A., Llanos-Muñoz M., et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): Results from the National Cancer Registry of Spain (RGETNE) Ann. Oncol. 2010;21:1794–1803. doi: 10.1093/annonc/mdq022. - DOI - PubMed
-
- Faggiano A., Ferolla P., Grimaldi F., Campana D., Manzoni M., Davì M.V., Bianchi A., Valcavi R., Papini E., Giuffrida D., et al. Natural history of gastro-entero-pancreatic and thoracic neuroendocrine tumors. Data from a large prospective and retrospective Italian Epidemiological study: THE NET MANAGEMENT STUDY. J. Endocrinol. Investig. 2011;35:817–823. - PubMed
-
- Maasberg S., Pape U.F., Fottner C., Goretzki P.E., Anlauf M., Hoersch D., Cremer B., Schulte D.M., Quietzsch D., Scheerer F., et al. Neuroendocrine Neoplasia within the German NET Registry. Z. Gastroenterol. 2018;56:1237–1246. - PubMed
LinkOut - more resources
Full Text Sources

