PET-CT in Clinical Adult Oncology-V. Head and Neck and Neuro Oncology
- PMID: 35681709
- PMCID: PMC9179458
- DOI: 10.3390/cancers14112726
PET-CT in Clinical Adult Oncology-V. Head and Neck and Neuro Oncology
Abstract
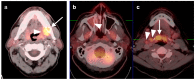
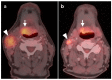
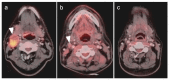
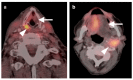
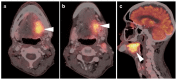
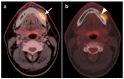
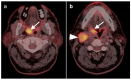
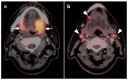
PET-CT is an advanced imaging modality with many oncologic applications, including staging, assessment of response to therapy, restaging, and longitudinal surveillance for recurrence. The goal of this series of six review articles is to provide practical information to providers and imaging professionals regarding the best use of PET-CT for specific oncologic indications, and the potential pitfalls and nuances that characterize these applications. In addition, key tumor-specific clinical information and representative PET-CT images are provided to outline the role that PET-CT plays in the management of oncology patients. Hundreds of different types of tumors exist, both pediatric and adult. A discussion of the role of FDG PET for all of these is beyond the scope of this review. Rather, this series of articles focuses on the most common adult malignancies that may be encountered in clinical practice. It also focuses on FDA-approved and clinically available radiopharmaceuticals, rather than research tracers or those requiring a local cyclotron. The fifth review article in this series focuses on PET-CT imaging in head and neck tumors, as well as brain tumors. Common normal variants, key anatomic features, and benign mimics of these tumors are reviewed. The goal of this review article is to provide the imaging professional with guidance in the interpretation of PET-CT for the more common head and neck malignancies and neuro oncology, and to inform the referring providers so that they can have realistic expectations of the value and limitations of PET-CT for the specific type of tumor being addressed.
Keywords: CNS lymphoma; FDG; PET; brain tumors; cervical esophageal cancer; encephalitis; head and neck cancer; leptomeningeal carcinomatosis; meningioma; salivary tumors; squamous cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


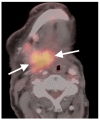
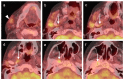
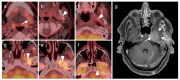
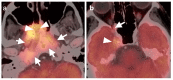
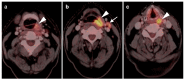

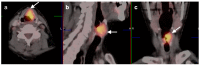
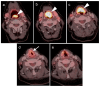
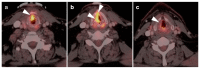
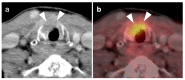
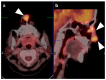
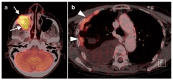
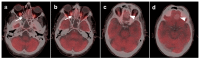




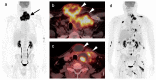

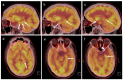




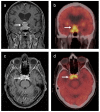
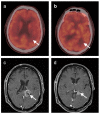
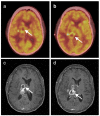
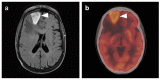
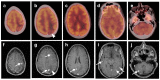
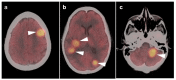
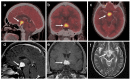

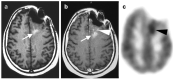
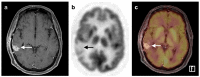
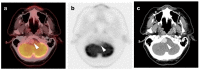


References
-
- Rodrigues R.S., Bozza F.A., Christian P.E., Hoffman J.M., Butterfield R.I., Christensen C.R., Heilbrun M., Wiggins R.H., Hunt J.P., Bentz B.G., et al. Comparison of whole-body PET/CT, dedicated high-resolution head and neck PET/CT, and contrast-enhanced CT in preoperative staging of clinically M0 squamous cell carcinoma of the head and neck. J. Nucl. Med. 2009;50:1205–1213. doi: 10.2967/jnumed.109.062075. - DOI - PubMed
-
- Cacicedo J., Navarro A., Del Hoyo O., Gomez-Iturriaga A., Alongi F., Medina J.A., Elicin O., Skanjeti A., Giammarile F., Bilbao P., et al. Role of fluorine-18 fluorodeoxyglucose PET/CT in head and neck oncology: The point of view of the radiation oncologist. Br. J. Radiol. 2016;89:20160217. doi: 10.1259/bjr.20160217. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

