Inhibitors of the Actin-Bundling Protein Fascin-1 Developed for Tumor Therapy Attenuate the T-Cell Stimulatory Properties of Dendritic Cells
- PMID: 35681718
- PMCID: PMC9179534
- DOI: 10.3390/cancers14112738
Inhibitors of the Actin-Bundling Protein Fascin-1 Developed for Tumor Therapy Attenuate the T-Cell Stimulatory Properties of Dendritic Cells
Abstract
Background: Stimulated dendritic cells (DCs), which constitute the most potent population of antigen-presenting cells (APCs), express the actin-bundling protein Fascin-1 (Fscn1). In tumor cells, de novo expression of Fscn1 correlates with their invasive and metastatic properties. Therefore, Fscn1 inhibitors have been developed to serve as antitumor agents. In this study, we were interested in better understanding the impact of Fscn1 inhibitors on DCs.
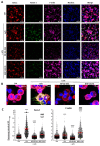
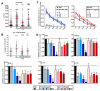
Methods: In parallel settings, murine spleen cells and bone-marrow-derived DCs (BMDCs) were stimulated with lipopolysaccharide in the presence of Fscn1 inhibitors (NP-G2-044 and BDP-13176). An analysis of surface expression of costimulatory and coinhibitory receptors, as well as cytokine production, was performed by flow cytometry. Cytoskeletal alterations were assessed by confocal microscopy. The effects on the interactions of BMDCs with antigen-specific T cells were monitored by time lapse microscopy. The T-cell stimulatory and polarizing capacity of BMDCs were measured in proliferation assays and cytokine studies.
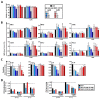
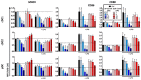
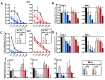
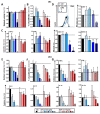
Results: Administration of Fscn1 inhibitors diminished Fscn1 expression and the formation of dendritic processes by stimulated BMDCs and elevated CD273 (PD-L2) expression. Fscn1 inhibition attenuated the interaction of DCs with antigen-specific T cells and concomitant T-cell proliferation.
Conclusions: Systemic administration of Fscn1 inhibitors for tumor therapy may also modulate DC-induced antitumor immune responses.
Keywords: BDP-13176; CD273; CD86; Fascin-1; NP-G2-044; dendritic cells.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Fascin actin-bundling protein 1 in human cancer: promising biomarker or therapeutic target?Mol Ther Oncolytics. 2021 Jan 20;20:240-264. doi: 10.1016/j.omto.2020.12.014. eCollection 2021 Mar 26. Mol Ther Oncolytics. 2021. PMID: 33614909 Free PMC article. Review.
-
Comprehensive Transcriptome and Pathway Analyses Revealed Central Role for Fascin in Promoting Triple-Negative Breast Cancer Progression.Pharmaceuticals (Basel). 2021 Nov 26;14(12):1228. doi: 10.3390/ph14121228. Pharmaceuticals (Basel). 2021. PMID: 34959629 Free PMC article.
-
CD40-CD40L cross-talk drives fascin expression in dendritic cells for efficient antigen presentation to CD4+ T cells.Int Immunol. 2017 Mar 1;29(3):121-131. doi: 10.1093/intimm/dxx013. Int Immunol. 2017. PMID: 28369442 Free PMC article.
-
Roles of Fascin in Dendritic Cells.Cancers (Basel). 2023 Jul 20;15(14):3691. doi: 10.3390/cancers15143691. Cancers (Basel). 2023. PMID: 37509352 Free PMC article. Review.
-
Phenotypic profile of dendritic and T cells in the lymph node of Balb/C mice with breast cancer submitted to dendritic cells immunotherapy.Immunol Lett. 2016 Sep;177:25-37. doi: 10.1016/j.imlet.2016.07.009. Epub 2016 Jul 14. Immunol Lett. 2016. PMID: 27423825
Cited by
-
Transcriptional Targeting of Dendritic Cells Using an Optimized Human Fascin1 Gene Promoter.Int J Mol Sci. 2023 Nov 29;24(23):16938. doi: 10.3390/ijms242316938. Int J Mol Sci. 2023. PMID: 38069260 Free PMC article.
-
Upregulated PCAT-1 predicts poor prognosis and reduced immune cell infiltration in head and neck squamous cell carcinoma through the miR-145-5p / FSCN-1 axis.Mol Biol Rep. 2025 Jan 13;52(1):121. doi: 10.1007/s11033-024-10208-1. Mol Biol Rep. 2025. PMID: 39806246
-
Targeting FSCN1 with an oral small-molecule inhibitor for treating ocular neovascularization.J Transl Med. 2023 Aug 18;21(1):555. doi: 10.1186/s12967-023-04225-0. J Transl Med. 2023. PMID: 37596693 Free PMC article.
References
-
- Mohr C.F., Gross C., Bros M., Reske-Kunz A.B., Biesinger B., Thoma-Kress A.K. Regulation of the tumor marker Fascin by the viral oncoprotein Tax of human T-cell leukemia virus type 1 (HTLV-1) depends on promoter activation and on a promoter-independent mechanism. Virology. 2015;485:481–491. doi: 10.1016/j.virol.2015.08.025. - DOI - PubMed
-
- Mosialos G., Yamashiro S., Baughman R.W., Matsudaira P., Vara L., Matsumura F., Kieff E., Birkenbach M. Epstein-Barr virus infection induces expression in B lymphocytes of a novel gene encoding an evolutionarily conserved 55-kilodalton actin-bundling protein. J. Virol. 1994;68:7320–7328. doi: 10.1128/jvi.68.11.7320-7328.1994. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

