The Role of Indoleamine 2, 3-Dioxygenase 1 in Regulating Tumor Microenvironment
- PMID: 35681736
- PMCID: PMC9179436
- DOI: 10.3390/cancers14112756
The Role of Indoleamine 2, 3-Dioxygenase 1 in Regulating Tumor Microenvironment
Abstract
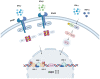
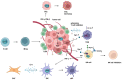
Indoleamine 2, 3-dioxygenase 1 (IDO1) is a rate-limiting enzyme that metabolizes an essential amino acid tryptophan (Trp) into kynurenine (Kyn), and it promotes the occurrence of immunosuppressive effects by regulating the consumption of Trp and the accumulation of Kyn in the tumor microenvironment (TME). Recent studies have shown that the main cellular components of TME interact with each other through this pathway to promote the formation of tumor immunosuppressive microenvironment. Here, we review the role of the immunosuppression mechanisms mediated by the IDO1 pathway in tumor growth. We discuss obstacles encountered in using IDO1 as a new tumor immunotherapy target, as well as the current clinical research progress.
Keywords: dendritic cell; indoleamine 2,3-dioxygenase 1; interferon-γ; myeloid-derived suppressor cell; regulatory T cell; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Up-regulation of indoleamine 2,3-dioxygenase 1 (IDO1) expression and catalytic activity is associated with immunosuppression and poor prognosis in penile squamous cell carcinoma patients.Cancer Commun (Lond). 2020 Jan;40(1):3-15. doi: 10.1002/cac2.12001. Epub 2020 Mar 3. Cancer Commun (Lond). 2020. PMID: 32125093 Free PMC article.
-
Indoleamine 2, 3-Dioxygenase 1 Mediates Survival Signals in Chronic Lymphocytic Leukemia via Kynurenine/Aryl Hydrocarbon Receptor-Mediated MCL1 Modulation.Front Immunol. 2022 Mar 18;13:832263. doi: 10.3389/fimmu.2022.832263. eCollection 2022. Front Immunol. 2022. PMID: 35371054 Free PMC article.
-
Indoleamine 2,3-Dioxygenase Activity-Induced Acceleration of Tumor Growth, and Protein Kinases-Related Novel Therapeutics Regimens.Adv Exp Med Biol. 2021;1275:339-356. doi: 10.1007/978-3-030-49844-3_13. Adv Exp Med Biol. 2021. PMID: 33539022
-
Dual-target inhibitors of indoleamine 2, 3 dioxygenase 1 (Ido1): A promising direction in cancer immunotherapy.Eur J Med Chem. 2022 Aug 5;238:114524. doi: 10.1016/j.ejmech.2022.114524. Epub 2022 Jun 8. Eur J Med Chem. 2022. PMID: 35696861 Review.
-
Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy - Challenges and Opportunities.Trends Pharmacol Sci. 2018 Mar;39(3):307-325. doi: 10.1016/j.tips.2017.11.007. Epub 2017 Dec 15. Trends Pharmacol Sci. 2018. PMID: 29254698 Review.
Cited by
-
Quantitative proteomics analysis of triple-negative breast cancers.NPJ Precis Oncol. 2025 Apr 24;9(1):117. doi: 10.1038/s41698-025-00907-8. NPJ Precis Oncol. 2025. PMID: 40269124 Free PMC article.
-
How to enhance MSCs therapeutic properties? An insight on potentiation methods.Stem Cell Res Ther. 2024 Sep 27;15(1):331. doi: 10.1186/s13287-024-03935-6. Stem Cell Res Ther. 2024. PMID: 39334487 Free PMC article. Review.
-
Combined Inhibition of Indolamine-2,3-Dioxygenase 1 and C-X-C Chemokine Receptor Type 2 Exerts Antitumor Effects in a Preclinical Model of Cervical Cancer.Biomedicines. 2023 Aug 16;11(8):2280. doi: 10.3390/biomedicines11082280. Biomedicines. 2023. PMID: 37626777 Free PMC article.
-
Harnessing IDO inhibitors to optimize cancer immunotherapy.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul 17. doi: 10.1007/s00210-025-04445-9. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40676292 Review.
-
Indoleamine 2, 3-dioxygenase 1 inhibitory compounds from natural sources.Front Pharmacol. 2022 Nov 4;13:1046818. doi: 10.3389/fphar.2022.1046818. eCollection 2022. Front Pharmacol. 2022. PMID: 36408235 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

