The Kynurenine Pathway and Cancer: Why Keep It Simple When You Can Make It Complicated
- PMID: 35681770
- PMCID: PMC9179486
- DOI: 10.3390/cancers14112793
The Kynurenine Pathway and Cancer: Why Keep It Simple When You Can Make It Complicated
Abstract
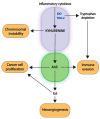
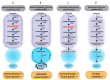
The kynurenine pathway has been highlighted as a gatekeeper of immune-privileged sites through its ability to generate from tryptophan a set of immunosuppressive metabolic intermediates. It additionally constitutes an important source of cellular NAD+ for the organism. Hijacking of its immunosuppressive functions, as recurrently observed in multiple cancers, facilitates immune evasion and promotes tumor development. Based on these observations, researchers have focused on characterizing indoleamine 2,3-dioxygenase (IDO1), the main enzyme catalyzing the first and limiting step of the pathway, and on developing therapies targeting it. Unfortunately, clinical trials studying IDO1 inhibitors have thus far not met expectations, highlighting the need to unravel this complex signaling pathway further. Recent advances demonstrate that these metabolites additionally promote tumor growth, metastatic dissemination and chemoresistance by a combination of paracrine and autocrine effects. Production of NAD+ also contributes to cancer progression by providing cancer cells with enhanced plasticity, invasive properties and chemoresistance. A comprehensive survey of this complexity is challenging but necessary to achieve medical success.
Keywords: NAD; kynurenine; tumor initiation and progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

