Cancer-Associated Fibroblasts Confer Gemcitabine Resistance to Pancreatic Cancer Cells through PTEN-Targeting miRNAs in Exosomes
- PMID: 35681792
- PMCID: PMC9179363
- DOI: 10.3390/cancers14112812
Cancer-Associated Fibroblasts Confer Gemcitabine Resistance to Pancreatic Cancer Cells through PTEN-Targeting miRNAs in Exosomes
Abstract
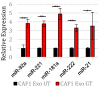
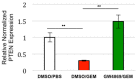
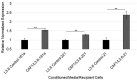
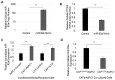
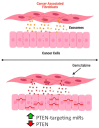
Pancreatic ductal adenocarcinoma (PDAC) is currently the third leading cause of cancer-related death in the United States. Even though the poor prognosis of PDAC is often attributed to late diagnosis, patients with an early diagnosis who undergo tumor resection and adjuvant chemotherapy still show tumor recurrence, highlighting a need to develop therapies which can overcome chemoresistance. Chemoresistance has been linked to the high expression of microRNAs (miRs), such as miR-21, within tumor cells. Tumor cells can collect miRs through the uptake of miR-containing lipid extracellular vesicles called exosomes. These exosomes are secreted in high numbers from cancer-associated fibroblasts (CAFs) within the tumor microenvironment during gemcitabine treatment and can contribute to cell proliferation and chemoresistance. Here, we show a novel mechanism in which CAF-derived exosomes may promote proliferation and chemoresistance, in part, through suppression of the tumor suppressor PTEN. We identified five microRNAs: miR-21, miR-181a, miR-221, miR-222, and miR-92a, that significantly increased in number within the CAF exosomes secreted during gemcitabine treatment which target PTEN. Furthermore, we found that CAF exosomes suppressed PTEN expression in vitro and that treatment with the exosome inhibitor GW4869 blocked PTEN suppression in vivo. Collectively, these findings highlight a mechanism through which the PTEN expression loss, often seen in PDAC, may be attained and lend support to investigations into the use of exosome inhibitors as potential therapeutics to improve the effectiveness of chemotherapy.
Keywords: PTEN; exosomes; fibroblasts; miRNAs; pancreatic cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cancer-associated fibroblasts suppress ferroptosis and induce gemcitabine resistance in pancreatic cancer cells by secreting exosome-derived ACSL4-targeting miRNAs.Drug Resist Updat. 2023 May;68:100960. doi: 10.1016/j.drup.2023.100960. Epub 2023 Mar 28. Drug Resist Updat. 2023. PMID: 37003125
-
Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells.Oncogene. 2017 Mar 30;36(13):1770-1778. doi: 10.1038/onc.2016.353. Epub 2016 Sep 26. Oncogene. 2017. PMID: 27669441 Free PMC article.
-
Downregulated exosomal microRNA-148b-3p in cancer associated fibroblasts enhance chemosensitivity of bladder cancer cells by downregulating the Wnt/β-catenin pathway and upregulating PTEN.Cell Oncol (Dordr). 2021 Feb;44(1):45-59. doi: 10.1007/s13402-020-00500-0. Epub 2021 Jan 10. Cell Oncol (Dordr). 2021. PMID: 33423167 Free PMC article.
-
Potential Role of Exosomes in the Chemoresistance to Gemcitabine and Nab-Paclitaxel in Pancreatic Cancer.Diagnostics (Basel). 2022 Jan 23;12(2):286. doi: 10.3390/diagnostics12020286. Diagnostics (Basel). 2022. PMID: 35204377 Free PMC article. Review.
-
The Role of Exosomes in Pancreatic Ductal Adenocarcinoma Progression and Their Potential as Biomarkers.Cancers (Basel). 2023 Mar 15;15(6):1776. doi: 10.3390/cancers15061776. Cancers (Basel). 2023. PMID: 36980662 Free PMC article. Review.
Cited by
-
Cancer-associated fibroblasts and its derived exosomes: a new perspective for reshaping the tumor microenvironment.Mol Med. 2023 May 22;29(1):66. doi: 10.1186/s10020-023-00665-y. Mol Med. 2023. PMID: 37217855 Free PMC article. Review.
-
Exosomes: Emerging Insights into the Progression of Pancreatic Cancer.Int J Biol Sci. 2024 Aug 1;20(10):4098-4113. doi: 10.7150/ijbs.97076. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113699 Free PMC article. Review.
-
Knowledge mapping and research trends of exosomes in pancreatic cancer: a bibliometric analysis and review (2013-2023).Front Oncol. 2024 Apr 24;14:1362436. doi: 10.3389/fonc.2024.1362436. eCollection 2024. Front Oncol. 2024. PMID: 38720811 Free PMC article. Review.
-
A spheroid whole mount drug testing pipeline with machine-learning based image analysis identifies cell-type specific differences in drug efficacy on a single-cell level.BMC Cancer. 2024 Dec 18;24(1):1542. doi: 10.1186/s12885-024-13329-9. BMC Cancer. 2024. PMID: 39696122 Free PMC article.
-
miRNAs in pancreatic cancer progression and metastasis.Clin Exp Metastasis. 2024 Jun;41(3):163-186. doi: 10.1007/s10585-023-10256-0. Epub 2024 Jan 19. Clin Exp Metastasis. 2024. PMID: 38240887 Free PMC article. Review.
References
-
- Society A.C. Cancer Facts and Figures. American Cancer Society; Atlanta, GA, USA: 2022.
-
- Oettle H., Post S., Neuhaus P., Gellert K., Langrehr J., Ridwelski K., Schramm H., Fahlke J., Zuelke C., Burkart C., et al. Adjuvant Chemotherapy With Gemcitabine vs. Observation in Patients Undergoing Curative-Intent Resection of Pancreatic Cancer. JAMA J. Am. Med Assoc. 2007;297:267–277. doi: 10.1001/jama.297.3.267. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

