The Activity of Plant-Derived Ren's Oligopeptides-1 against the Pseudorabies Virus
- PMID: 35681806
- PMCID: PMC9179334
- DOI: 10.3390/ani12111341
The Activity of Plant-Derived Ren's Oligopeptides-1 against the Pseudorabies Virus
Abstract
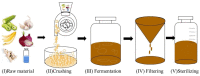
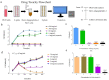
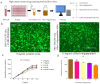
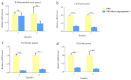
Newly synthesized Ren's oligopeptides-1 was found to have an antiviral effect in clinical trials, and the purpose of this study was to further demonstrate the antiviral activity of Ren's oligopeptides-1 against the PRV 152-GFP strain. We used the real-time cell analysis system (RTCA) to detect the cytotoxicity of different concentrations of Ren's oligopeptides-1. We then applied high content screening (HCS) to detect the antiviral activity of Ren's oligopeptides-1 against PRV. Meanwhile, the fluorescence signal of the virus was collected in real time and the expression levels of the related genes in the PK15 cells infected with PRV were detected using real-time PCR. At the mRNA level, we discovered that, at a concentration of 6 mg/mL, Ren's oligopeptides-1 reduced the expression of pseudorabies virus (PRV) genes such as IE180, UL18, UL54, and UL21 at a concentration of 6 mg/mL. We then determined that Ren's oligopeptides-1 has an EC50 value of 6 mg/mL, and at this level, no cytotoxicity was observed.
Keywords: RTCA; Ren’s oligopeptides-1; high content screening; pseudorabies virus (PRV).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

