Sulfur Amino Acid Metabolism and the Role of Endogenous Cystathionine-γ-lyase/H2S in Holstein Cows with Clinical Mastitis
- PMID: 35681915
- PMCID: PMC9179249
- DOI: 10.3390/ani12111451
Sulfur Amino Acid Metabolism and the Role of Endogenous Cystathionine-γ-lyase/H2S in Holstein Cows with Clinical Mastitis
Abstract
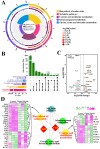
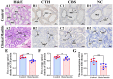
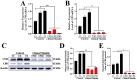
H2S plays an important role in various inflammatory diseases. However, the role of H2S and synthetic enzymes in Holstein cows with CM is unknown. The aim of this study was to identify DEPs associated with sulfide metabolism and further investigate their roles in dairy cows with CM. From 3739 DEPs generated by data-independent acquisition proteomics, we identified a total of 17 DEPs included in 44 GO terms and five KEGG pathways related to sulfide metabolism, including CTH and cystathionine-β-synthase (CBS). Immunohistochemical and immunofluorescence staining results showed that CTH and CBS proteins were present mainly in the cytoplasm of mammary epithelial cells. Endogenous H2S production in the serum of the CM group was significantly lower than that of the healthy Holstein cows. CTH and CBS mRNA and protein levels in the mammary glands of the CM group were significantly downregulated compared to those of the healthy group. These results indicate that CTH and H2S were correlated with the occurrence and development of CM in Holstein cows, which provides important insights into the function and regulatory mechanism of CTH/H2S in Holstein cows.
Keywords: clinical mastitis; cystathionine-γ-lyase; hydrogen sulfide; inflammation.
Conflict of interest statement
The authors declare that no conflict of interest exist.
Figures


Similar articles
-
CTH/H2S Regulates LPS-Induced Inflammation through IL-8 Signaling in MAC-T Cells.Int J Mol Sci. 2022 Oct 5;23(19):11822. doi: 10.3390/ijms231911822. Int J Mol Sci. 2022. PMID: 36233122 Free PMC article.
-
The H2S-generating enzymes cystathionine β-synthase and cystathionine γ-lyase play a role in vascular development during normal lung alveolarization.Am J Physiol Lung Cell Mol Physiol. 2015 Oct 1;309(7):L710-24. doi: 10.1152/ajplung.00134.2015. Epub 2015 Jul 31. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26232299
-
E2β stimulates ovine uterine artery endothelial cell H2S production in vitro by estrogen receptor-dependent upregulation of cystathionine β-synthase and cystathionine γ-lyase expression†.Biol Reprod. 2019 Feb 1;100(2):514-522. doi: 10.1093/biolre/ioy207. Biol Reprod. 2019. PMID: 30277497 Free PMC article.
-
Hydrogen Sulfide as a Potential Therapeutic Target in Fibrosis.Oxid Med Cell Longev. 2015;2015:593407. doi: 10.1155/2015/593407. Epub 2015 May 11. Oxid Med Cell Longev. 2015. PMID: 26078809 Free PMC article. Review.
-
[Cystathionine γ-lyase].Postepy Hig Med Dosw (Online). 2014 Jan 15;68:1-9. doi: 10.5604/17322693.1085372. Postepy Hig Med Dosw (Online). 2014. PMID: 24491890 Review. Polish.
Cited by
-
Toll-like Receptor 2 Is Associated with the Immune Response, Apoptosis, and Angiogenesis in the Mammary Glands of Dairy Cows with Clinical Mastitis.Int J Mol Sci. 2022 Sep 14;23(18):10717. doi: 10.3390/ijms231810717. Int J Mol Sci. 2022. PMID: 36142648 Free PMC article.
-
CTH/H2S Regulates LPS-Induced Inflammation through IL-8 Signaling in MAC-T Cells.Int J Mol Sci. 2022 Oct 5;23(19):11822. doi: 10.3390/ijms231911822. Int J Mol Sci. 2022. PMID: 36233122 Free PMC article.
-
HMOX1 Promotes Ferroptosis in Mammary Epithelial Cells via FTH1 and Is Involved in the Development of Clinical Mastitis in Dairy Cows.Antioxidants (Basel). 2022 Nov 11;11(11):2221. doi: 10.3390/antiox11112221. Antioxidants (Basel). 2022. PMID: 36421410 Free PMC article.
-
Proteomic Profiling of Hu Sheep Placental Development Across Gestational Stages Reveals Stage-Specific Regulatory Networks.Int J Mol Sci. 2025 Apr 29;26(9):4236. doi: 10.3390/ijms26094236. Int J Mol Sci. 2025. PMID: 40362472 Free PMC article.
-
Role of CORO1A in Regulating Immune Homeostasis of Mammary Glands and Its Contribution to Clinical Mastitis Development in Dairy Cows.Biomolecules. 2025 Jun 6;15(6):827. doi: 10.3390/biom15060827. Biomolecules. 2025. PMID: 40563467 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

