A Systematic Review and Meta-Analysis of Systemic Antibiotic Therapy in the Treatment of Peri-Implantitis
- PMID: 35682086
- PMCID: PMC9180155
- DOI: 10.3390/ijerph19116502
A Systematic Review and Meta-Analysis of Systemic Antibiotic Therapy in the Treatment of Peri-Implantitis
Abstract
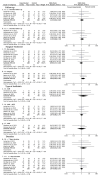
Research has been conducted into the advantages of the systemic administration of antibiotics. The aim of this systematic review and meta-analysis was to assess the efficacy of systemic antibiotic administration in the treatment of peri-implantitis in terms of bleeding on probing (BoP) and probing pocket depth (PPD). Literature searches were performed across PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) to identify randomized controlled trials and observational clinical studies. After peri-implantitis treatment, PPD was reduced by 0.1 mm (p = 0.58; IC 95% [-0.24, 0.47]), indicating a non-significant effect of antibiotic administration on PPD. The BoP odds ratio value was 1.15 (p = 0.5; IC 95% [0.75, 1.75]), indicating that the likelihood of bleeding is almost similar between the test and control groups. Secondary outcomes were found, such as reduced clinical attachment level, lower suppuration and recession, less bone loss, and a reduction in total bacterial counts. In the treatment of peri-implantitis, the systemic antibiotic application reduces neither PPD nor BoP. Therefore, the systemic administration of antibiotics, in the case of peri-implantitis, should be rethought in light of the present results, contributing to address the problem of increasing antibiotic resistance.
Keywords: antibacterial agents; antibiotic resistance; antibiotics; bleeding on probing; peri-implantitis; probing pocket depth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Deeb M.A., Alsahhaf A., Mubaraki S.A., Alhamoudi N., Al-Aali K.A., Abduljabbar T. Clinical and Microbiological Outcomes of Photodynamic and Systemic Antimicrobial Therapy in Smokers with Peri-Implant Inflammation. Photodiagnosis Photodyn. Ther. 2020;29:101587. doi: 10.1016/j.pdpdt.2019.101587. - DOI - PubMed
-
- Berglundh T., Armitage G., Araujo M.G., Avila-Ortiz G., Blanco J., Camargo P.M., Chen S., Cochran D., Derks J., Figuero E., et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018;45:S286–S291. doi: 10.1111/jcpe.12957. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

