Novel Cyclopentaquinoline and Acridine Analogs as Multifunctional, Potent Drug Candidates in Alzheimer's Disease
- PMID: 35682556
- PMCID: PMC9179981
- DOI: 10.3390/ijms23115876
Novel Cyclopentaquinoline and Acridine Analogs as Multifunctional, Potent Drug Candidates in Alzheimer's Disease
Abstract
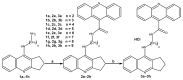
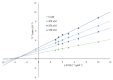
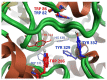
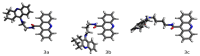
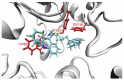
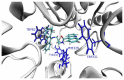
A series of new cyclopentaquinoline derivatives with 9-acridinecarboxylic acid and a different alkyl chain length were synthesized, and their ability to inhibit cholinesterases was evaluated. All designed compounds, except derivative 3f, exhibited a selectivity for butyrylcholinesterase (BuChE) with IC50 values ranging from 103 to 539 nM. The 3b derivative revealed the highest inhibitory activity towards BuChE (IC50 = 103.73 nM) and a suitable activity against AChE (IC50 = 272.33 nM). The 3f derivative was the most active compound to AChE (IC50 = 113.34 nM) with satisfactory activity towards BuChE (IC50 = 203.52 nM). The potential hepatotoxic effect was evaluated for both 3b and 3f compounds. The 3b and 3f potential antioxidant activity was measured using the ORAC-FL method. The 3b and 3f derivatives revealed a significantly higher antioxidant potency, respectively 35 and 25 higher than tacrine. Theoretical, physicochemical, and pharmacokinetic properties were calculated using ACD Labs Percepta software. Molecular modeling and kinetic study were used to reveal the mechanism of cholinesterase inhibition in the most potent compounds: 3b and 3f.
Keywords: Alzheimer’s disease; biological activity; multitarget small molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

