Mass Spectrometry-Based Disulfide Mapping of Lysyl Oxidase-like 2
- PMID: 35682561
- PMCID: PMC9180022
- DOI: 10.3390/ijms23115879
Mass Spectrometry-Based Disulfide Mapping of Lysyl Oxidase-like 2
Abstract
Lysyl oxidase-like 2 (LOXL2) catalyzes the oxidative deamination of peptidyl lysines and hydroxylysines to promote extracellular matrix remodeling. Aberrant activity of LOXL2 has been associated with organ fibrosis and tumor metastasis. The lysine tyrosylquinone (LTQ) cofactor is derived from Lys653 and Tyr689 in the amine oxidase domain via post-translational modification. Based on the similarity in hydrodynamic radius and radius of gyration, we recently proposed that the overall structures of the mature LOXL2 (containing LTQ) and the precursor LOXL2 (no LTQ) are very similar. In this study, we conducted a mass spectrometry-based disulfide mapping analysis of recombinant LOXL2 in three forms: a full-length LOXL2 (fl-LOXL2) containing a nearly stoichiometric amount of LTQ, Δ1-2SRCR-LOXL2 (SRCR1 and SRCR2 are truncated) in the precursor form, and Δ1-3SRCR-LOXL2 (SRCR1, SRCR2, SRCR3 are truncated) in a mixture of the precursor and the mature forms. We detected a set of five disulfide bonds that is conserved in both the precursor and the mature recombinant LOXL2s. In addition, we detected a set of four alternative disulfide bonds in low abundance that is not associated with the mature LOXL2. These results suggest that the major set of five disulfide bonds is retained post-LTQ formation.
Keywords: disulfide bonds; lysine tyrosylquinone; lysyl oxidase-like 2; mass spectrometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

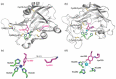

References
-
- Csiszar K. Lysyl oxidases: A novel multifunctional amine oxidase family. Prog. Nucleic Acid. Res. Mol. Biol. 2001;70:1–32. - PubMed
-
- Salvador F., Martin A., Lopez-Menendez C., Moreno-Bueno G., Santos V., Vazquez-Naharro A., Santamaria P.G., Morales S., Dubus P.R., Muinelo-Romay L., et al. Lysyl Oxidase-like Protein LOXL2 Promotes Lung Metastasis of Breast Cancer. Cancer Res. 2017;77:5846–5859. doi: 10.1158/0008-5472.CAN-16-3152. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

