A Potential Role of Keratinocyte-Derived Bilirubin in Human Skin Yellowness and Its Amelioration by Sucrose Laurate/Dilaurate
- PMID: 35682565
- PMCID: PMC9180758
- DOI: 10.3390/ijms23115884
A Potential Role of Keratinocyte-Derived Bilirubin in Human Skin Yellowness and Its Amelioration by Sucrose Laurate/Dilaurate
Abstract
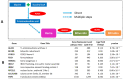
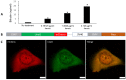
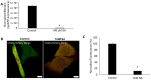
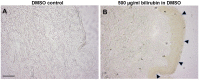
Sallow and/or dull skin appearance is greatly attributable to the yellow components of skin tone. Bilirubin is a yellow chromophore known to be made in the liver and/or spleen and is transported throughout the body via the blood stream. Recent publications suggest bilirubin may be synthesized in other cells/organs, including the skin. We found human keratinocytes express the transcripts involved in bilirubin biosynthesis. In parallel, we also found human keratinocytes could indeed synthesize bilirubin in monolayer keratinocytes and in a 3D human skin-equivalent model. The synthesized amount was substantial enough to contribute to skin yellowness. In addition, oxidative stress enhanced bilirubin production. Using UnaG, a protein that forms a fluorescent species upon binding to bilirubin, we also visualized the intracellular expression of bilirubin in keratinocytes. Finally, we screened a compound library and discovered that the sucrose laurate/dilaurate (SDL) combination significantly reduced bilirubin levels, as well as bilirubin-mediated yellowness. In conclusion, bilirubin is indeed synthesized in epidermal keratinocytes and can be upregulated by oxidative stress, which could contribute to chronic or transient yellow skin tone appearance. Application of SDL diminishes bilirubin generation and may be a potential solution to mitigate yellowish and/or dull skin appearance.
Keywords: bilirubin; keratinocyte; oxidative stress; skin color; skin tone; skin yellowness; sucrose dilaurate; sucrose laurate.
Conflict of interest statement
Bin Fang, Junjun Chen, Lijuan Li, Timothy Laughlin, Bradley Jarrold, Wenzhu Zhao, and Tomohiro Hakozaki are employees of the Procter & Gamble Company and contributed to the design of the study; to the collection, analyses, or interpretation of data; to the writing of the manuscript, and to the decision to publish the results.
Figures

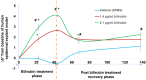
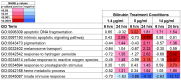
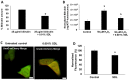
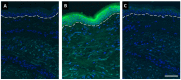
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

