Mitochondrial Dysfunction in Spinocerebellar Ataxia Type 3 Is Linked to VDAC1 Deubiquitination
- PMID: 35682609
- PMCID: PMC9180688
- DOI: 10.3390/ijms23115933
Mitochondrial Dysfunction in Spinocerebellar Ataxia Type 3 Is Linked to VDAC1 Deubiquitination
Abstract
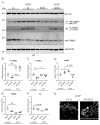
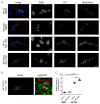
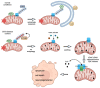
Dysfunctional mitochondria are linked to several neurodegenerative diseases. Metabolic defects, a symptom which can result from dysfunctional mitochondria, are also present in spinocerebellar ataxia type 3 (SCA3), also known as Machado-Joseph disease, the most frequent, dominantly inherited neurodegenerative ataxia worldwide. Mitochondrial dysfunction has been reported for several neurodegenerative disorders and ataxin-3 is known to deubiquitinylate parkin, a key protein required for canonical mitophagy. In this study, we analyzed mitochondrial function and mitophagy in a patient-derived SCA3 cell model. Human fibroblast lines isolated from SCA3 patients were immortalized and characterized. SCA3 patient fibroblasts revealed circular, ring-shaped mitochondria and featured reduced OXPHOS complexes, ATP production and cell viability. We show that wildtype ataxin-3 deubiquitinates VDAC1 (voltage-dependent anion channel 1), a member of the mitochondrial permeability transition pore and a parkin substrate. In SCA3 patients, VDAC1 deubiquitination and parkin recruitment to the depolarized mitochondria is inhibited. Increased p62-linked mitophagy, autophagosome formation and autophagy is observed under disease conditions, which is in line with mitochondrial fission. SCA3 fibroblast lines demonstrated a mitochondrial phenotype and dysregulation of parkin-VDAC1-mediated mitophagy, thereby promoting mitochondrial quality control via alternative pathways.
Keywords: Machado–Joseph disease; VDAC1 ubiquitination; ataxin-3; mitochondria dysfunction; spinocerebellar ataxia type 3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Chou A.-H., Yeh T.-H., Kuo Y.-L., Kao Y.-C., Jou M.-J., Hsu C.-Y., Tsai S.-R., Kakizuka A., Wang H.-L. Polyglutamine-expanded ataxin-3 activates mitochondrial apoptotic pathway by upregulating Bax and downregulating Bcl-xL. Neurobiol. Dis. 2006;21:333–345. doi: 10.1016/j.nbd.2005.07.011. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

