The Effects of Acidosis on eNOS in the Systemic Vasculature: A Focus on Early Postnatal Ontogenesis
- PMID: 35682667
- PMCID: PMC9180972
- DOI: 10.3390/ijms23115987
The Effects of Acidosis on eNOS in the Systemic Vasculature: A Focus on Early Postnatal Ontogenesis
Abstract
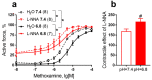
The activity of many vasomotor signaling pathways strongly depends on extracellular/intracellular pH. Nitric oxide (NO) is one of the most important vasodilators produced by the endothelium. In this review, we present evidence that in most vascular beds of mature mammalian organisms metabolic or respiratory acidosis increases functional endothelial NO-synthase (eNOS) activity, despite the observation that direct effects of low pH on eNOS enzymatic activity are inhibitory. This can be explained by the fact that acidosis increases the activity of signaling pathways that positively regulate eNOS activity. The role of NO in the regulation of vascular tone is greater in early postnatal ontogenesis compared to adulthood. Importantly, in early postnatal ontogenesis acidosis also augments functional eNOS activity and its contribution to the regulation of arterial contractility. Therefore, the effect of acidosis on total peripheral resistance in neonates may be stronger than in adults and can be one of the reasons for an undesirable decrease in blood pressure during neonatal asphyxia. The latter, however, should be proven in future studies.
Keywords: acidosis; anticontractile influence; eNOS; early postnatal development; endothelium; nitric oxide; pH; vasculature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Mateuszuk Ł., Campagna R., Kutryb-Zając B., Kuś K., Słominska E.M., Smolenski R.T., Chlopicki S. Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochem. Pharmacol. 2020;178:114019. doi: 10.1016/j.bcp.2020.114019. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

