Critical Review on Fatty Acid-Based Food and Nutraceuticals as Supporting Therapy in Cancer
- PMID: 35682708
- PMCID: PMC9181022
- DOI: 10.3390/ijms23116030
Critical Review on Fatty Acid-Based Food and Nutraceuticals as Supporting Therapy in Cancer
Abstract
Fatty acids have an important place in both biological and nutritional contexts and, from a clinical point of view, they have known consequences for diseases' onset and development, including cancer. The use of fatty acid-based food and nutraceuticals to support cancer therapy is a multidisciplinary subject, involving molecular and clinical research. Knowledge regarding polyunsaturated fatty acids essentiality/oxidizability and the role of lipogenesis-desaturase pathways for cell growth, as well as oxidative reactivity in cancer cells, are discussed, since they can drive the choice of fatty acids using their multiple roles to support antitumoral drug activity. The central role of membrane fatty acid composition is highlighted for the application of membrane lipid therapy. As fatty acids are also known as biomarkers of cancer onset and progression, the personalization of the fatty acid-based therapy is also possible, taking into account other important factors such as formulation, bioavailability and the distribution of the supplementation. A holistic approach emerges combining nutra- and pharma-strategies in an appropriate manner, to develop further knowledge and applications in cancer therapy.
Keywords: anticancer strategy; dietary fatty acids; fatty acid signaling; membrane fatty acids; membrane lipidomics; precision nutraceuticals.
Conflict of interest statement
C.F. and C.C. are co-founders of the spin-off company Lipinutragen srl (Bologna, Italy) which is involved in membrane lipidomics. Lipinutragen had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
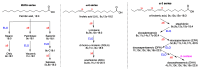
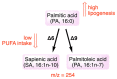
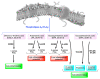
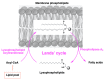
References
-
- Ferreri C., Masi A., Sansone A., Giacometti G., Larocca A., Menounou G., Scanferlato R., Tortorella S., Rota D., Chatgilialoglu C. Fatty acids in Membranes as Homeostatic, Metabolic and Nutritional Biomarkers: Recent Advancements in Analytics and Diagnostics. Diagnostics. 2017;7:1. doi: 10.3390/diagnostics7010001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

