Altered Brain Arginine Metabolism and Polyamine System in a P301S Tauopathy Mouse Model: A Time-Course Study
- PMID: 35682712
- PMCID: PMC9181759
- DOI: 10.3390/ijms23116039
Altered Brain Arginine Metabolism and Polyamine System in a P301S Tauopathy Mouse Model: A Time-Course Study
Abstract
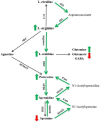
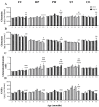
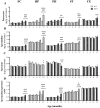
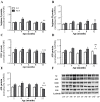
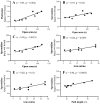
Altered arginine metabolism (including the polyamine system) has recently been implicated in the pathogenesis of tauopathies, characterised by hyperphosphorylated and aggregated microtubule-associated protein tau (MAPT) accumulation in the brain. The present study, for the first time, systematically determined the time-course of arginine metabolism changes in the MAPT P301S (PS19) mouse brain at 2, 4, 6, 8 and 12 months of age. The polyamines putrescine, spermidine and spermine are critically involved in microtubule assembly and stabilization. This study, therefore, further investigated how polyamine biosynthetic and catabolic enzymes changed in PS19 mice. There were general age-dependent increases of L-arginine, L-ornithine, putrescine and spermidine in the PS19 brain (particularly in the hippocampus and parahippocampal region). While this profile change clearly indicates a shift of arginine metabolism to favor polyamine production (a polyamine stress response), spermine levels were decreased or unchanged due to the upregulation of polyamine retro-conversion pathways. Our results further implicate altered arginine metabolism (particularly the polyamine system) in the pathogenesis of tauopathies. Given the role of the polyamines in microtubule assembly and stabilization, future research is required to understand the functional significance of the polyamine stress response and explore the preventive and/or therapeutic opportunities for tauopathies by targeting the polyamine system.
Keywords: PS19 mice; arginine; hippocampus; polyamine; polyamine stress response; spermine; tau; tauopathy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript or in the decision to publish the results.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

